Abstract
During endovascular aneurysm repair (EVAR), interruption of the internal iliac arteries (IIAs) or the inferior mesenteric artery by stents or embolization is thought to cause colon ischemia. To minimize this risk, attempts have been made to preserve the IIAs using iliac branch devices or IIA revascularization. Here we present our experience of colon ischemia after EVAR in a patient with bilaterally patent IIAs without evidence of embolism. A 70-year-old man had abdominal pain and a ruptured abdominal aortic aneurysm was found. We performed EVAR with custom-made tube grafts preserving the bilateral IIAs. On postoperative day 2, the patient complained of abdominal pain, a sigmoidoscopy was performed revealing colon ischemia. On laparotomy, transmural infarction of the sigmoid colon was found and resected. Because IIA preservation cannot guarantee protection against colon ischemia, surgeons should maintain a high level of suspicion and use surveillance liberally after EVAR for early diagnosis of colon ischemia, even if both IIAs are preserved.
Left colon ischemia after both open and endovascular aneurysm repair (EVAR) is well documented. The incidence of clinically significant colonic ischemia has been reported to be as high as 6% after EVAR, and the mortality rate associated with transmural bowel necrosis is high [1]. Because direct visual assessment of colonic perfusion, which is possible with open repair, is not available to the endovascular surgeons, determination of perioperative risk factors as predictors of ischemia is of critical importance. Proposed major causes of colon ischemia after EVAR include interruption of the inferior mesenteric artery (IMA) and internal iliac arteries (IIAs), and embolization [1]. Many endovascular surgeons worry about colon ischemia when bilateral IIA occlusion is necessary during EVAR to treat concomitant bilateral common iliac aneurysms. Bilateral IIA embolization has been reported to result in a high incidence of pelvic ischemic symptoms. Among these, buttock claudication and colonic ischemia are two common examples. Theoretically, ischemic complications may be reduced in patients who receive IIA revascularization, IIA preservation through an iliac branch device, or sequential embolization to enhance collateral flow across the pelvis [2]. However, there are little data to support a direct association between the proposed risk factors and colonic ischemia, and contradictory results have been reported for the impact of IIA exclusion on colonic perfusion [3].
Atheroembolism has been implicated as the major etiology of colon ischemia in patients with preserved bilateral IIA circulation [4]. Here, we present a case of colonic ischemia after EVAR in a patient with bilaterally patent IIAs without evidence of embolism.
A 70-year-old man presented with acute abdominal pain and was diagnosed with a ruptured infrarenal abdominal aortic aneurysm (AAA). On arrival, he was alert and oriented, and his blood pressure was 148/78 mmHg. He had hypertension and end-stage renal disease and was receiving regular hemodialysis. He had a history of a cerebrovascular accident 23 years prior and acute myocardial infarction 12 years prior, both of which were treated by percutaneous transluminal coronary angioplasty
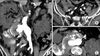
A computed tomography (CT) scan showed a 38-mm infrarenal abdominal aortic aneurysm without iliac artery dilation and a hematoma that was confined to the retroperitoneal cavity around the aneurysm. The aneurysm ended 18 mm proximal to the iliac bifurcation. IMA, both IIAs, and the celiac and superior mesenteric artery were patent (Fig. 1). The aorta and the iliac arteries were severely calcified. An arteriogram was performed with the patient under general anesthesia, and leakage of contrast accompanying a drop in blood pressure to 70/45 mmHg was observed. Tube grafts (28 × 80 mm, 26 × 60 mm; SEAL, S&G Biotech Inc., Seongnam, Korea) were inserted through the right femoral artery. Because we deployed the wider graft first, there was a significant amount of endoleak between the two different size endografts. We deployed another tube graft (28 × 80 mm, SEAL, S&G Biotech Inc.) between the two other tube grafts, thereby successfully excluding the AAA with complete preservation of both iliac arteries. On the second postoperative day, the patient complained of diffuse abdominal pain and his C-reactive protein level was elevated at 31.18 mg/dL. Sigmoidoscopy revealed severe ischemic colitis from 20 cm proximal to the anal verge to the sigmoid colon. On laparotomy, there was transmural ischemia and infarction of the sigmoid colon, but no evidence of perforation (Fig. 2A). A colonic resection with formation of Hartmann's pouch and colostomy was performed (Fig. 2B). Pathologic examination revealed transmural infarction without evidence of atheroembolism. Postoperatively, it was difficult to wean the patient off the ventilator, he remained in the intensive care unit for two months and underwent a tracheostomy. He was discharged from the hospital three months after surgery.
Colon ischemia is the most common manifestation of ischemic injury to the gastrointestinal tract. Diminution of the colonic blood supply that is so severe that metabolic demands cannot be met results from anatomic occlusion or a decrease in systemic perfusion. In the setting of EVAR, the precise etiology of colon ischemia is still unclear, though the most commonly appraised risk factors related to anatomy are IMA and IIA occlusion. During EVAR, sacrifice of the IMA is universal, and the ability to assess ischemia by direct examination of the colon is lost, as is the option of reimplantation of the IMA. However, studies that evaluated the influence of ligation or replantation of the IMA revealed that these factors were not significantly associated with the risk of developing perioperative ischemic colitis [5]. Furthermore, the incidence of colon ischemia after open AAA repair has been reported to be significantly higher than that after endovascular repair [6].
Preservation of the IIAs as an important collateral supply to the colon after EVAR can potentially be used to reduce the incidence of colon ischemia. However, the role of IIA occlusion in the development of colon ischemia has been a subject of some controversy, with some studies reporting that interruption of one IIA or even both IIAs is not a major cause of colon ischemia in the setting of EVAR [7]. One study suggested that branches of the ipsilateral EIA provide a more significant collateral pathway than the contralateral IIA [8].
As a non-anatomic factor, systemic hypoperfusion is also responsible for triggering a mesenteric vasoconstrictive reflex and subsequent development of colon ischemia [9]. Intraoperative hemodynamic disturbances and blood loss associated with ruptured AAA are associated with this condition.
In our case, preservation of both IIAs did not prevent colon ischemia. A recent report on colon ischemia after using an iliac branch device is consistent with our experience [2]. There was no hematoma inside or nearby the mesentery of the sigmoid colon that could have compressed feeding vessels on laparotomy, and there was no evidence of atheroembolism in the pathologic specimens. Although we cannot draw firm conclusions from our case alone, hypoperfusion of the left colon due to systemic hypotension may contribute more to ischemic colitis than occlusion of IIAs. When considering preventive actions, attempts to maintain hemodynamic stability during and after the procedure should be prioritized because many patients experience colonic hypoperfusion for the first few hours after surgery.
A long operation time due to persistent endoleaks between endografts could also cause colon ischemia due to intraoperative hemodynamic disturbances and blood loss, as suggested previously [10]. In our patient, we did not take the mismatch in diameters of the grafts into account during the emergency procedure and deployed the wider graft first. This resulted in a significant amount of endoleak between the two endografts, and the operation lasted more than three hours. Therefore, because IIA preservation is not globally protective against colon ischemia, surgeons should maintain a high level of suspicion and use surveillance techniques such as endoscopy after EVAR liberally to facilitate early diagnosis of colonic ischemia in high-risk patients, even if both IIAs are preserved.
Figures and Tables
References
1. Dadian N, Ohki T, Veith FJ, Edelman M, Mehta M, Lipsitz EC, et al. Overt colon ischemia after endovascular aneurysm repair: the importance of microembolization as an etiology. J Vasc Surg. 2001. 34:986–996.
2. Sfyroeras GS, Koutsias S, Karathanos C, Stamoulis K, Giannoukas AD. Colonic ischemia after endovascular exclusion of an aortoiliac aneurysm using an iliac branch device. Vasc Endovascular Surg. 2010. 44:597–600.
3. Maldonado TS, Rockman CB, Riles E, Douglas D, Adelman MA, Jacobowitz GR, et al. Ischemic complications after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2004. 40:703–709.
4. Geraghty PJ, Sanchez LA, Rubin BG, Choi ET, Flye MW, Curci JA, et al. Overt ischemic colitis after endovascular repair of aortoiliac aneurysms. J Vasc Surg. 2004. 40:413–418.
5. Senekowitsch C, Assadian A, Assadian O, Hartleb H, Ptakovsky H, Hagmüller GW. Replanting the inferior mesentery artery during infrarenal aortic aneurysm repair: influence on postoperative colon ischemia. J Vasc Surg. 2006. 43:689–694.
6. Perry RJ, Martin MJ, Eckert MJ, Sohn VY, Steele SR. Colonic ischemia complicating open vs endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2008. 48:272–277.
7. Bratby MJ, Munneke GM, Belli AM, Loosemore TM, Loftus I, Thompson MM, et al. How safe is bilateral internal iliac artery embolization prior to EVAR? Cardiovasc Intervent Radiol. 2008. 31:246–253.
8. Iliopoulos JI, Hermreck AS, Thomas JH, Pierce GE. Hemodynamics of the hypogastric arterial circulation. J Vasc Surg. 1989. 9:637–641.
9. Elder K, Lashner BA, Al Solaiman F. Clinical approach to colonic ischemia. Cleve Clin J Med. 2009. 76:401–409.
10. Björck M, Troëng T, Bergqvist D. Risk factors for intestinal ischaemia after aortoiliac surgery: a combined cohort and case-control study of 2824 operations. Eur J Vasc Endovasc Surg. 1997. 13:531–539.




 ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download