Abstract
Purpose
Band erosion is a well-known complication of laparoscopic adjustable gastric band placement. We gained experience with laparoscopic removal of an eroded gastric band.
Methods
We retrospectively reviewed the operative log of our obesity surgery unit to identify all operations performed for band erosion from March 2009 to May 2011.
Results
During the study period, a total of six of 96 patients (6.3%), five females and one male, were diagnosed with band erosion and underwent surgical removal of the band system. The median time interval from the initial gastric band placement to the diagnosis of band erosion was 8.5 months (range, 7 to 22 months), with most band erosion occurring within the first year (5/6, 83%). The median body mass index at band removal was 28.4 kg/m2. Upper abdominal pain was the most common symptom (5/6, 83%), and other signs and symptoms were port site infection (3/6, 50%) and loss of restriction and weight regain (1/6, 17%). All eroded bands were removed using laparoscopy. Further complications after laparoscopic removal of the band system were observed in three cases. One patient showed multiple intra-abdominal abscesses requiring insertion of a pigtail catheter for drainage. The other two patients experienced sepsis with localized peritonitis, eventually requiring laparoscopic washout and drainage.
Conclusion
Gastric band erosion requires the removal of the gastric band. Laparoscopic removal is technically achievable in the majority of patients with eroded gastric band. The method can be challenging, has potential postoperative complications (fistula, abscess), and should be attempted only by experienced surgeons.
Laparoscopic adjustable gastric banding (LAGB) is one of the most popularly performed bariatric surgical procedures worldwide. Its advantages include adjustability, relative ease of performance, acceptable weight loss effect, and the low perioperative complication rate. According to the literature, the medium-term excess weight loss ranges from 50 to 70% [1-6]. However, in the long term, a small group of patients who undergo LAGB suffer from complications such as band slippage, pouch dilatation, prosthesis infection, and band erosion (BE). Unlike other complications, BE always requires removal of the band system because long-standing inflammation associated with the eroded gastric band has proven to be associated with a variety of serious morbidities [7-11]. Controversy exists regarding the management of a patient with an eroded gastric band. In this paper, we aimed to present our experience with diagnosis and the surgical removal of an eroded gastric band.
From March 2009 to May 2011, 96 morbidly obese patients (85 females and 11 males) with an average age of 33.6 years (range, 18 to 58 years) underwent LAGB. The LAGB procedure was performed as described previously [12]. In brief, the port positions included a 15-mm umbilical port for the camera and two 5-mm ports, one in each subcostal area, for the acting instruments. A Nathanson liver retractor (Cook Medical, Queensland, Australia) was inserted through a 5-mm skin incision in the subxiphoid location and curved upward to retract the left hepatic lobe. We used a pars flaccida technique of dissection in all cases, and two or three gastrogastric sutures were inserted using 2-0 Ethibond (Ethicon Inc., Somerville, NJ, USA). After LAGB, all band adjustments were performed in a fluoroscopy room in our surgical office. A barium swallow test was also performed whenever there were clinical suspicions of BE (i.e., unusual abdominal pain, port site infection, loss of restriction and weight regain). Abdominal computed tomography (CT) scan was performed when the barium swallow test showed abnormal flow patterns compatible with BE. It was also performed if the patient was considered to have localized peritonitis or a septic focus associated with BE, and esophagogastroduodenoscopy (EGD) was performed to confirm the diagnosis of BE. Based on the EGD findings, the degree of migration was classified according to Nocca et al. [13] as follows; stage I, a small part of the band visible through a hole in the gastric mucosa; stage II, partial migration (>50% of the band free in the gastric lumen), and stage III, complete migration. The previous laparoscopic incisions were used for laparoscopic re-exploration in all patients. We carefully dissected and identified the lap-band tubing up to the band buckle, then debuckled the locking system using a laparoscopic grasper and removed the band system. Four steps were performed for safe closure of the remaining gastric perforation after band removal; 1) primary repair (PR): using 2-0 Ethibond (Ethicon Inc.), an interrupted suture was used to close the defect, 2) omental plugging (OP): segmentation of the vascularized omentum was fashioned and gently inserted through the tunnel (i.e., that leads into the stomach) that is usually left behind by the extracted band and fixed in place using multiple sutures through a relatively healthy gastric wall [14], 3) drainage catheter insertion (DR): one subhepatic (at the level of suture line) and one left subphrenic Jackson-Pratt (JP) drain were inserted through a left subcostal incision, and 4) nasogastric tube insertion (NT): a nasogastric tube was inserted for decompression of the air and drainage of gastric juice, and the tube was maintained for postoperative 48 hours. Patients with documented BE requiring surgical re-intervention were identified from a database and case notes were reviewed. We retrospectively reviewed the demographic findings (age, gender, preoperative body mass index [BMI], comorbidities), band, and port fixation technique of each patient. We also reviewed the time interval between BE diagnosis and initial LAGB placement, BMI at band removal, associated clinical signs and symptoms, band filling volume, stage and site of BE, operative findings at the time of band removal, further complications and operative management, and length of hospital stay.
A 21-year-old female patient with BMI of 41.9 kg/m2 underwent an uneventful LAGB (Lap-Band 10.0, Allegan Inc., Irvine, CA, USA). The patient had shown good weight loss with up to postoperative 20 months. She complained gradual loss of restriction and weight regain (+10 kg from the nadir body weight). The barium swallow test showed no abnormal findings. EGD a showed BE in the left inferior banding site. The patient underwent an uneventful laparoscopic band removal.
A 18-year-old female patients with BMI of 33.9 kg/m2 (her comorbidities were dyslipidemia, non-alcoholic fatty liver disease [NAFLD], and polycystic ovary syndrome [PCOS]), underwent an uneventful LAGB (Lap-Band 9.75, Allegan Inc.). On the 15th postoperative day, the patient showed signs of port infection, which was treated with local wound care and healed uneventfully. Her weight loss was excellent. At postoperative 9 months, her BMI was 21.9 kg/m2. The patient showed a recurrent port infection associated with left upper abdominal pain, which was not subsided with conservative management. The barium swallow test did not show abnormal findings. Abdominal CT scan showed small collection of air around the band. EGD confirmed the BE. The patient underwent a laparoscopic band removal (omental patch technique). After band removal, the patient became severely septic from the first postoperative day, and follow-up abdominal CT scan showed multiple intraabdominal abscesses. Six pigtail catheters were inserted for drainage via a modified Seldinger technique. The recovery of the patient required a prolonged period of total parenteral nutrition and intravenous (IV) antibiotic treatment. Length of hospital stay was 74 days.
A 47-year-old female patient with BMI of 35.2 kg/m2 (her comorbidities were dyslipidemia and NAFLD) underwent an uneventful LAGB (Lap-Band 9.75, Allegan Inc.). After operation, the patient was satisfied with the gradual weight loss, and did not show up to the hospital for band adjustment. At postoperative 8 months, the patient complained about sudden redness and swelling of port site and severe upper mid-abdominal pain. Local wound exploration showed greenish mucous collection around the port site. EGD confirmed the BE. The patient underwent an uneventful band removal surgery.
A 46-year-old male patient with BMI of 37.2 kg/m2 (his comorbidities were hypertension, variant angina) underwent an uneventful LAGB (Swedish adjustable gastric band, SAGB; Ethicon Endo-Surgery Inc., Cincinnati, Ohio, USA). At postoperative 3 months, he showed mild port site infection which was treated by local wound care, and at postoperative 8 months, port infection was redeveloped, and he complained intermittent severe abdominal pain around the port site. The barium swallow test did not show abnormal findings. Infection and abdominal pain were relieved with local wound treatment. However at postoperative 9 months abdominal pain recurred. He also complained dysphagia on liquid as well as solid food. An EGD diagnosed BE. Five days after laparoscopic band removal, the patient showed localized peritonitis in the right lower quadrant (RLQ) abdomen. Abdominal CT scan showed multifocal, loculated, and encapsulated fluid collection in the RLQ abdomen (diameter: 6.5 cm), eventually requiring laparoscopic washout and drainage.
A 36-year-old female patient with BMI of 36.7 kg/m2 underwent an uneventful LAGB (Lap-Band APS, Allergan Inc.). She had showed good weight loss until postoperative 6 months, when she developed fever and left upper quadrant abdominal pain not relieved by analgesics and oral antibiotics. The barium swallow test showed barium flow outside of the normal band stoma. Abdominal CT scan showed left subphrenic abscesses originating from gastric microperforation due to BE. However EGD did not showed a definite mucosal defect. As the symptoms were relieved by conservative treatment during the admission, future endoscopic band removal was suggested. After one month, however, she redeveloped spiking fever, pyrexia, and severe myalgia. Follow-up abdominal CT scan showed a huge intrahepatic abscess within the left lobe of the liver (8 cm in diameter). Emergent laparoscopic band removal was performed. Intraoperative EGD showed a small mucosal defect in the left inferior banding site.
A 35-year-old female patient with her BMI of 41.5 kg/m2 underwent an uneventful LAGB (Lap-Band, Allergan Inc.). At postoperative 7 months, she developed severe abdominal pain from epigastrium to right lower quadrant abdomen. The barium swallow test and abdominal CT scan showed findings compatible with BE. On EGD mucosal erosion lesion was located in the posterosuperior banding area. On the third days after laparoscopic band removal, the patient showed a spiking fever, tachycardia, and left pleuritic chest pain. Abdominal CT scan showed a suspicious perigastric abscess near the suture line and loculated fluid collections or abscess indenting left lateral segment of liver, eventually requiring laparoscopic washout and drainage.
During the study period, six of 96 patients (6.3%), five females and one male, were diagnosed with BE and underwent surgical removal of the band system. The mean age of six patients at the time of LAGB placement was 33.8 years, and their average pre-LAGB BMI was 37.7 kg/m2 (range, 33.9 to 41.9 kg/m2). Four patients had comorbidities; four required subcutaneous ports in the anterior rectus fascia, and two had subfascial ports as described previously [15] (Table 1). The median time interval from the initial LAGB placement to the diagnosis of BE was 8.5 months (range, 7 to 22 months), with most BE occurring within the first year after initial LAGB placement (5/6, 83%). The median BMI at band removal was 28.4 kg/m2, thus the average of BMI change (rapidity of weight loss) of these patients and the non-complicated patients at postoperative 8.5 months was 8.4 and 7.2, respectively. Upper abdominal pain was the most common symptom (5/6, 83%), and the other signs and symptoms were port site infection (3/6, 50%) and loss of restriction and weight regain (1/6, 17%). As far as diagnosis of erosion is concerned, a total of eight patients underwent abdominal CT scan. Among the 96 patients, a total of 13 patients underwent study for questionable signs and symptoms suggestive of BE. Two of 13 patients showed barium flow outside the normal band stoma (patients #5, 6). Both patients underwent an abdominal CT scan, which showed localized small collections of the air around the silicon band suggestive of microperforations of the stomach. Eleven patients showed normal barium passage along the band stoma. Six of them underwent abdominal CT scans for further evaluation of their persistent abdominal discomfort. Among these 6 patients, 2 patients were diagnosed to have BE. Abdominal CT scan was positive in one patient (patient #2), and negative in the other patient (patient #3). The other five patients did not undergo abdominal CT scan. Among them, two patients were diagnosed to have BE. These two patients were diagnosed to have BE by EGD (patients #1, 4) (Fig. 1). The most common erosion site was the left inferior banding site (4/6, 66.7%) (Fig. 2). All cases of BE were grade I and were treated via laparoscopic removal of the band system. Further complications after laparoscopic removal of the band system were observed in three patients. One patient (patient #2) showed fever and suspicious sepsis on the first day after band removal. Five days after the surgery, the patient required the insertion of multiple pigtail catheters for multiple intra-abdominal abscesses. The recovery of the patient required a prolonged period of total parenteral nutrition and IV antibiotic treatment. The other two patients (patients #4, 6) had a fever and localized peritonitis after band removal, eventually requiring laparoscopic washout and drainage (Fig. 3). The median length of hospital stay (LOS) for all patients was 9.5 days (range, 4 to 74 days), and there was no mortality (Table 2). All of the patients who underwent removal of eroded gastric band started to regain their weight after one month. All revisional operations were performed by the patients' requests. Revisional laparoscopic sleeve gastrectomy was performed in patients #1 and 4. Second laparoscopic gastric band was inserted in patients #2 and 6. As the patients did not want more surgery, no more bariatric procedure was performed in patients #3 and 5.
BE is a major complication of LAGB, requiring removal of the gastric band system. According to the literature, BE after LAGB occurs in about 0.5 to 11% of cases [16,17]. In this study, six of 96 patients (6.3%) were diagnosed with BE and underwent surgical removal of the band system, which is a slightly higher incidence than in other reported series. It is well known that there is a significant correlation between BE rate and surgeon experience. The annual risk of BE during the first two years of surgical practice is much higher than those of subsequent years [18]. Data from a systemic review of BE also showed that the rate of erosion is as high as 17% in reports involving less than 100 patients, and the rate of erosion decreases over time [19]. Therefore, the erosion in our series of patients actually occurred in the learning phase. However, definite cause of BE is yet to be determined. Current understanding is that BE is initiated by acute and chronic tissue damage. In this regards, we should perform pars flaccida technique accurately to avoid entering the lesser sac, and injuring the posterior gastric wall, which could minimize the occurrence of erosion. Gastrogastric suture should be performed to minimize tissue damage in making the fundal wrap, and therefore to avoid BE. As chronic ischemic tissue damage is regarded as possible cause of erosion, excessive band filling should be avoided (currently, we do not fill the band more than 2 mL at one time). All these technical considerations could minimize the occurrence of BE in our recent patients. Second, we routinely conduct radiologic band adjustment using fluoroscopy for all patients underwent gastric banding surgery. Therefore, we are able to detect a small barium leakage outside the stoma. This policy might differ from those of other institutions with a higher threshold for endoscopy performance. Third, we preferred 'bolus' filling for optimal band adjustment in patients who showed suboptimal weight loss because it is more focused on adjusting the 'stomal diameter'. In general, band-filling starts 6 to 8 weeks postoperatively with a total of three to six adjustments during the first year. This stepwise filling strategy is used with caution and is based on the intention to enable sufficient healing of the band device within the gastro-gastric tunnel [20]. However, there is no scientific data on the effects of the different filling protocols on the incidence of BE. Usually, we fill the band gradually (at least 2-week interval) for postoperative 2 to 3 months to reach the "Green zone" (optimal restriction). One time filling volume is less than 1.5 mL. However, if weight loss is not sufficient or unsatisfactory (e.g., less than 0.5 kg per week), we adopt 'bolus' filling protocol, that is, we fill the band at one time until appropriate radiologic appearance meet our criteria (stoma diameter less than 3 mm, mild to moderate barium reflux). In this circumstance, one time filling volume is occasionally more than 2.0 mL. The clinical manifestations of BE can range from asymptomatic to acute peritonitis and sepsis. Occasionally, BE is undetected until found at diagnostic laparoscopy. Asymptomatic individuals can often increase their food intake and gain weight. In our series of patients, abdominal pain was the predominant symptom, which is in line with findings from other studies [18]. In our series of patients with BE, 83% complained of upper abdominal pain, and port infection was present in 50%. There are several potential causes of abdominal pain after LAGB placement. For example, abdominal pain can be caused by pouch distention, small bowel ileus, constipation, overeating, and peritoneal irritation of the connecting tube. However, abdominal pain associated with BE is constant and is not related to eating. It usually starts in the epigastrium and may radiate to the upper back, left subcostal area, and under the breast bone. As time passes, bacterial inflammation originates from the 'gastric perforation' in BE and migrates along the connecting tube due to the dense adhesive capsule formed by the chronic foreign body reaction. Therefore, patients complain of intermittent, severe pain in the lower abdomen. When re-laparoscopy is performed, localized abscesses or inflammatory adhesions along the band system are often observed. Port site infection is quite bothersome for both clinicians and patients because it does not respond to local wound treatment such as drainage and antibiotics. The relatively poor immunologic ability of the subcutaneous fat layer to adequately handle the presence of even small amounts of gastric secretions results in chronic irritation, which eventually results in infection and often presents as wound breakdown. Of note, the two patients who had a subfascial port did not present with port infection even in the presence of BE, which is one of the advantages of subfascial port implantation. Port site infection and breakdown originated from BE is quite annoying for both patients and clinicians, and therefore it hinders conservative treatment and postponement of band removal surgery when the BE does occur. We agree with Clough et al. [15] in that subfascial space is resistant to sepsis. It can be explained by sufficient vascularity of the surrounding muscles originating from the superior and inferior epigastric arterial branches. Actually in terms of the subfascial space being resistant to sepsis, this is just a theory based on the observation that we have had no infections from subfascial port placement - particularly notable after previous infections where the port was removed from the standard position and placed subfascially at a later date without further infection setting in. We wonder whether many port infections are due to infected hematoma from the subcutaneous dissection required in the obese patient to place the port. Perhaps the fascia separates the port from this and from the skin where superficial infection may arise. It is just a theory and our numbers are far too small unfortunately to adequately examine this outcome properly. With regard to erosion, we have seen many recurrent port infections in the setting of BE (presumably infection tracking down the tubing) but because we have never had a subfascial port become infected it is hard to comment on the relationship between erosion and subfascial infection (so called 'fasciitis'). Another clinical manifestation of the patients with BE in this study was a relatively higher filling volume. The average filling volume of patients who did not have BE was 2.8 ± 0.9 with a 4 mL band and 5.9 ± 2.0 with a 10 mL band. Based on the clinical signs and symptoms suggestive of BE, upper gastrointestinal (UGI) and abdominal CT scan are helpful. The barium swallow test typically shows barium passing from the upper to the lower gastric pouch outside the band. Abdominal CT scan can detect small free air or localized abscess formation around the band, tube, and reservoir port. EGD is the definitive study instrument of choice. On EGD, the band typically protrudes into the gastric lumen. However, special attention should be paid to the identification of small erosive lesions just beneath the gastroesophageal (GE) junction and around the corners of the fundal wrap and bulged gastric mucosa with no visible erosion. Occasionally, pus (infected purulent fluid) descending down from the LAGB "pouch" near the GE junction may be the only sign of BE on retroversion at UGI endoscopy [18]. BE is due to multifactorial causes. It has been generally accepted that early BE is related to micro-injury during operation, and late BE is related to foreign body responses. However, the etiology of BE is still obscure. Our cohort of patients had relatively early erosion around the first postoperative year, and therefore small erosion below 25% of the total band area, and all but one presented with symptoms of inflammation. A potential source of BE is injury during surgery. For example, the laparoscopic grasper or band passer can damage the posterior wall of the stomach during band placement. In this series, all but one erosion occurred on the left side of the band, where the gastrogastric sutures were placed. We agree with Niville et al. [21] in that the chronic ischemic change caused by too tight and excessively deep stitches or a tight band tunnel may result in BE. Usually, mucosal defect in BE is observed in the left inferior banding site, where gastrogastric sutures are placed and pressure from filled band is maximal. Occasionally, on screening EGD (as many Korean people underwent a screening EGD annually), we could observe small stitch inside the gastric lumen in asymptomatic patients. We have also observed friable mucosa like mosaic ('snakeskin') pattern on endoscopy in the left inferior banding site in asymptomatic patients. All these are actually pre-erosion. Therefore, we recommend three important technical considerations during gastrogastric suture to minimize tissue damage in making the fundal wrap, and therefore to avoid BE. First, the sutures should be placed superficially and should not enter the gastric mucosa. Second, the stitch should not be tied too tightly. Third, the band tunnel created by pulling the fundus should be sufficiently large to avoid overstretching during adjustment. There is no consensus on the technique of removal of the eroded gastric band. In this series of patients, all eroded bands were removed via direct dissection outside the stomach because we decided that the band was easily identified along the connecting tube following dissection. However, the fibrous capsule around the band is actually 'unhealthy' phlegmonous tissue. Therefore, after removal of the band, closure of the remaining gastric perforation is very difficult, and repaired gastrostomies are more prone to leakage and breakdown. In our series, three patients suffered from postoperative complications after band removal. In this regard, transgastric removal, which is laparoscopic gastrostomy with intraluminal division and removal of the eroded band, seems to be a more relevant procedure [22]. Recent reports also suggest that endoscopic removal of the eroded band is a feasible procedure [23-27]. Transgastric techniques are possible only if a considerable portion of the eroded band is located in the gastric lumen, when the band is not easily identified outside the stomach following a dissection along the connecting tube. Endoscopic removal of an eroded band may require multiple sessions [28], and patient symptoms might prevent postponement of the procedure while waiting for the erosion to progress. For example, one of patients in this study (patient #5) was diagnosed with small, shallow erosion, and we planned a future endoscopic removal. However, while waiting, the patient developed a spiking fever, pyrexia, and multiple intrahepatic abscesses, which required urgent surgical removal. Therefore, the endoscopic approach should only be indicated in asymptomatic patients. Most cases of BE in our series were early with small erosion (grade I), and well-developed symptoms. As described above, the band was easily identified outside the stomach following dissection along the connecting tube. In this situation, we think an extragastric approach is more effective. Fistula and leakage after removal of the eroded bands were serious complications in our series. However, as shown in two cases (patients #3, 5), no postoperative complications developed after codification of techniques for band removal. Another important consideration should be suture materials. We have used 2-0 Ethibond (Ethicon Inc.) suture materials in gastric banding surgery mainly because it can hold the tissue permanently. When we repair the perforation site after removal of the eroded band, we feel it technically difficult to perform a tension-free repair of perforation site. In this regards, we think 2-0 Ethibond ideal to repair the perforation site. Two important technical considerations in repair of the perforation site are - 1) healthy gastric wall should be included in the suturing, 2) suture material should not enter the mucosa layer. In general, absorbable suture material is preferred in GI suture and monofilament suture material is preferred in order to prevent tissue damage when the tissue is friable. It would be valuable to compare the surgical outcome of removal of eroded band according to the suture material to close the gastric perforation. Based on our experience, we highly recommend the use of the four steps described below for safe closure of the defect after removal of eroded gastric band: 1) primary repair: an interrupted non-absorbable suture should be used to close the defect, 2) omental plugging: segmentation of the vascularized omentum is fashioned and gently inserted through the tunnel that is usually left behind by the extracted band and fixed in place using multiple sutures through a relatively healthy gastric wall, 3) drainage catheter insertion: one subhepatic (at the level of suture line) and one left subphrenic JP drain should be inserted through a left subcostal incision, and 4) nasogastric tube insertion: a nasogastric tube should be inserted for decompression of the air and drainage of gastric juice, and the tube should be maintained for postoperative 48 hours.
In conclusion, gastric BE requires the removal of the gastric band. Laparoscopic removal is technically achievable in the majority of patients with eroded gastric band. The method can be challenging, has potential postoperative complications (fistula, abscess), and should be attempted only by experienced surgeons.
Figures and Tables
 | Fig. 1Flow chart showing the diagnosis procedure in patients with band erosion (BE). UGI, upper gastrointestinal; CT, computed tomography; EGD, esophagogastroduodenoscopy. |
 | Fig. 2Location of band erosion on esophagogastroduodenoscopy. 1, anterosuperior; 2, posterosuperior; 3, anteroinferior; 4, posteroinferior. |
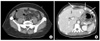 | Fig. 3(A) Five days after band removal, the patient (#4) showed localized peritonitis in the right lower quadrant (RLQ) abdomen. Abdominal computed tomopgraphy (CT) scan showed multifocal, loculated, and encapsulated fluid collection in the RLQ (diameter: 6.5 cm) (arrow). (B) Third days after band removal, the patient (#6) showed a spiking fever, tachycardia, and left pleuritic chest pain. Abdominal CT scan showed a suspicious perigastric abscess near the suture line and loculated fluid collections or abscess indenting anterior surface of the left lateral segment of liver (long arrows). A closed-suction drain was located in the subhepatic space (short arrows). |
Table 1
Patient demographics, comorbidities, and port fixation techniques at the time of LAGB placement

Table 2
Overview of patients who underwent laparoscopic removal of the migrated gastric band

Pt, patient; BE, band erosion; BMI, body mass index; PR, primary repair; OP, omental plugging; DR, drain catheter insertion; NT, nasogastric tube insertion for postoperative 48 hours; LOS, length of hospital stay.
a)Vol means band filling volume when patient was diagnosed with BE. b)Mucous collection at the port site. c)The degree of migration was classified as suggested by Nocca et al. [13]
References
1. Cunneen SA, Phillips E, Fielding G, Banel D, Estok R, Fahrbach K, et al. Studies of Swedish adjustable gastric band and Lap-Band: systematic review and meta-analysis. Surg Obes Relat Dis. 2008. 4:174–185.
2. O'Brien PE, Brown WA, Smith A, McMurrick PJ, Stephens M. Prospective study of a laparoscopically placed, adjustable gastric band in the treatment of morbid obesity. Br J Surg. 1999. 86:113–118.
3. Favretti F, Enzi G, Pizzirani E, Segato G, Belluco C, Pigato P, et al. Adjustable silicone gastric banding (ASGB): the Italian experience. Obes Surg. 1993. 3:53–56.
4. Parikh MS, Fielding GA, Ren CJ. U.S. experience with 749 laparoscopic adjustable gastric bands: intermediate outcomes. Surg Endosc. 2005. 19:1631–1635.
5. Vertruyen M. Experience with Lap-band System up to 7 years. Obes Surg. 2002. 12:569–572.
6. O'Brien PE, Dixon JB, Brown W, Schachter LM, Chapman L, Burn AJ, et al. The laparoscopic adjustable gastric band (Lap-Band): a prospective study of medium-term effects on weight, health and quality of life. Obes Surg. 2002. 12:652–660.
7. Egbeare DM, Myers AF, Lawrance RJ. Small bowel obstruction secondary to intragastric erosion and migration of a gastric band. J Gastrointest Surg. 2008. 12:983–984.
8. Rao AD, Ramalingam G. Exsanguinating hemorrhage following gastric erosion after laparoscopic adjustable gastric banding. Obes Surg. 2006. 16:1675–1678.
9. Campos J, Ramos A, Galvão Neto M, Siqueira L, Evangelista LF, Ferraz A, et al. Hypovolemic shock due to intragastric migration of an adjustable gastric band. Obes Surg. 2007. 17:562–564.
10. Wylezol M, Sitkiewicz T, Gluck M, Zubik R, Pardela M. Intra-abdominal abscess in the course of intragastric migration of an adjustable gastric band: a potentially life-threatening complication. Obes Surg. 2006. 16:102–104.
11. De Roover A, Detry O, Coimbra C, Hamoir E, Honoré P, Meurisse M. Pylephlebitis of the portal vein complicating intragastric migration of an adjustable gastric band. Obes Surg. 2006. 16:369–371.
12. Fielding GA, Allen JW. A step-by-step guide to placement of the LAP-BAND adjustable gastric banding system. Am J Surg. 2002. 184:26S–30S.
13. Nocca D, Frering V, Gallix B, de Seguin des Hons C, Noël P, Foulonge MA, et al. Migration of adjustable gastric banding from a cohort study of 4236 patients. Surg Endosc. 2005. 19:947–950.
14. Cherian PT, Goussous G, Sigurdsson A. Management of band erosion with omental plugging: case series from a 5-year laparoscopic gastric banding experience. Obes Surg. 2009. 19:1409–1413.
15. Clough A, Layani L, Sidhu M, Wheatley L, Shah A. Subfascial port placement in gastric banding surgery. Obes Surg. 2011. 21:604–608.
16. Abu-Abeid S, Szold A. Results and complications of laparoscopic adjustable gastric banding: an early and intermediate experience. Obes Surg. 1999. 9:188–190.
17. Silecchia G, Restuccia A, Elmore U, Polito D, Perrotta N, Genco A, et al. Laparoscopic adjustable silicone gastric banding: prospective evaluation of intragastric migration of the lap-band. Surg Laparosc Endosc Percutan Tech. 2001. 11:229–234.
18. Cherian PT, Goussous G, Ashori F, Sigurdsson A. Band erosion after laparoscopic gastric banding: a retrospective analysis of 865 patients over 5 years. Surg Endosc. 2010. 24:2031–2038.
19. Egberts K, Brown WA, O'Brien PE. Systematic review of erosion after laparoscopic adjustable gastric banding. Obes Surg. 2011. 21:1272–1279.
20. Kirchmayr W, Klaus A, Mühlmann G, Mittermair R, Bonatti H, Aigner F, et al. Adjustable gastric banding: assessment of safety and efficacy of bolus-filling during follow-up. Obes Surg. 2004. 14:387–391.
21. Niville E, Dams A, Vlasselaers J. Lap-Band erosion: incidence and treatment. Obes Surg. 2001. 11:744–747.
22. Basa NR, Dutson E, Lewis C, Derezin M, Han S, Mehran A. Laparoscopic transgastric removal of eroded adjustable band: a novel approach. Surg Obes Relat Dis. 2008. 4:194–197.
23. Regusci L, Groebli Y, Meyer JL, Walder J, Margalith D, Schneider R. Gastroscopic removal of an adjustable gastric band after partial intragastric migration. Obes Surg. 2003. 13:281–284.
24. Weiss H, Nehoda H, Labeck B, Peer R, Aigner F. Gastroscopic band removal after intragastric migration of adjustable gastric band: a new minimal invasive technique. Obes Surg. 2000. 10:167–170.
25. Fonnest GF, Jess P. Gastroscopic removal of penetrating gastric band. Ugeskr Laeger. 1999. 161:5930–5931.
26. Doğan ÜB, Akova A, Solmaz S, Aydin M. Gastroscopic removal of a migrated adjustable gastric band: a case report. Turk J Gastroenterol. 2010. 21:297–301.
27. Neto MP, Ramos AC, Campos JM, Murakami AH, Falcão M, Moura EH, et al. Endoscopic removal of eroded adjustable gastric band: lessons learned after 5 years and 78 cases. Surg Obes Relat Dis. 2010. 6:423–427.
28. Campos JM, Evangelista LF, Neto MP, Pagnossin G, Fernandes A, Ferraz AA, et al. Translumenal endoscopic drainage of abdominal abscess due to early migration of adjustable gastric band. Obes Surg. 2010. 20:247–250.




 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download