Abstract
Purpose
Although local resection like endoscopic mucosal resection for early gastric cancer is accepted as a treatment option, one of the most important drawbacks of such an approach is the inability to predictlymph node metastasis. The aim of this study was to evaluate the serum soluble receptor alpha for interleukin-2 (IL-2Rα) level as a predictor of lymph node metastasis in the patients with early gastric cancer.
Methods
Assessment of pre-operative serum IL-2Rα levels was performed on 86 patients with early gastric cancer treated by gastrectomies combined with D2 lymph node resections and 20 healthy controls at Samsung Medical Center. Data on patient age and gender, tumor size, depth of invasion, histologic differentiation, and endoscopic findings were reviewed post-operatively. The submucosal lesions were divided into three layers (sm1, sm2, and sm3) in accordance with the depth of invasion.
The gastric cancer mortality rate has been decreasing worldwide, but remains the second leading cause of cancer-related deaths. In an attempt to improve the quality of life in patients undergoing early gastric cancer treatment, endoscopic mucosal resection (EMR), laparoscopic-assisted gastrectomy, and robotic surgery have been introduced [1-3]. According to the pretreatment cancer stage, we can be choosing optimal treatment modality. When Following EMR, the permanent pathologic report indicates the tumor has invaded the submucosal layer, or tumor differentiation was confirmed to be an undifferentiated type, there is a high risk of lymph node metastasis and further treatment may be necessary, including lymph node dissection [4]. Indeed, lymph node metastasis is the most significant risk factor for recurrence and survival [5]. If we had known the lymph node metastasis before EMR, we must choose other treatment modality which related radical surgery with lymph node dissection (even D2 is possible) such as laparoscopic-assisted gastrectomy, or robotic surgery.
There are many diagnostic tools for lymph node metastasis in gastric cancer, endoscopic ultrasonography and computed tomography (CT) is used for prediction of lymph node metastasis, but the specificity of any pre-operative diagnostic tool is limited [6]. Thus, additional methods to predict lymph node metastasis would be useful in determining what types of treatment methods to be applied. Specifically, an easily measureable serum cytokine which predicted lymph node metastasis in early gastric cancer patients would be an important diagnostic tool.
Smith [7] reported that interleukin (IL)-2 stimulates macrophages in a similar manner to helper T-cells, cytotoxic T-cells, B-cells, and natural killer cells.
When IL-2 receptor alpha (IL-2Rα) is activated, the soluble form is released into the serum, thus we can assess the level of IL-2Rα in the serum. In colorectal and breast cancer patients, an elevation in the serum IL-2Rα level indicates disease progression to stage IV or liver metastasis with colorectal cancer, and distant metastasis in breast cancer [8]. However, in gastric cancer patients, an elevation of serum IL-2Rα level is associated with stage progression, but in case of lymph node metastasis such association was not well established.
Thus, we attempted to identify the relationship between the pre-operative serum IL-2Rα level and lymph node metastasis, and thereby assess the use of the serum IL-2Rα level as a predictor of lymph node metastasis, and consequently as a prognostic indicator in patients with early gastric cancer.
From May through December 2003, 86 patients who were diagnosed with early gastric cancer and underwent surgery at the Samsung Medical Center were enrolled as volunteers in the current study. Of the 86 patients, 66 were confirmed by pathology to have tumor invasion of the submucosal layer, while another 20 patients had invasion of the mucosal layer. These patients included 52 males and 34 females, with an average age of 57.5 years (range, 28 to 74 years).
Pre-operative endoscopy was used to assess the morphology of the tumors and the results of pre-operative abdominal computer tomography were reviewed for all patients. Patients were divided into two groups based on tumor invasion into mucosal and submucosal groups, and the submucosal groups was further divided into sm1, sm2, and sm3 according to the depth of tumor invasion, based on post-operative pathology. Papillary, well and moderately differentiated adenocarcinoma were classified as 'differentiated types,' while poorly differentiated adenocarcinoma, mucinous adenocarcinoma, and signet ring cell carcinoma were classified as 'undifferentiated types.' The results obtained from these patients were compared with 20 healthy individuals. Ethical approval was given by the Internal Review Board prior to commencing this study.
Before each surgery, serum was collected from each patient and stored at -80℃ until assay. The levels of serum-soluble IL-2Rα were measured by an ELISA using an interleukin-2 receptor kit (R&D Systems Inc., Minneapolis, MN, USA) in accordance with the manufacturer's instructions. The control group for the measurement of serum-soluble IL-2Rα levels consisted of 20 healthy individuals. The method used a four-fold dilution of patient serum (50 µL sample + 150 µL calibrator diluent RD6S) compared against standards and controls applied to a 96-well plate, which was coated with monoclonal antibodies against IL-2Ra.
The standards were diluted to 5,000, 2,500, 1,250, 625, 312, and 78 pg/mL, then the secondary antibody was applied. The assay was performed at room temperature (25℃) and incubated for 3 hours. The plates were washed four times, followed by incubation with a combined substrate solution consisting of tetrametyl benzidine and hydrogen peroxide. After a 20 minutes incubation in the dark, substrate stopping solution was added and the absorbance was measured at 450 nm. Each assay was performed in triplicate.
All statistical analyses were performed using the SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA). Using the measured values, a receiver operatiing characteristic (ROC) curve was performed and a cut-off value was determined, which produced high specificity and high sensitivity. The risk factors of lymph node metastasis were investigated by univariate and multivariate analyses using a two-tailed chi-square test and logistic regression. The significance of the difference amongst means was determined by a Student's t-test. A P-value < 0.05 was considered significant.
Among the 86 patients assessed, 16 had lymph node metastases (18.6%). The mean serum level of IL-2Rα in patients with gastric cancer was 222.79 ± 98.1 U/mL, which was significantly higher than the normal controls (131.96 ± 95.5 U/mL; P = 0.0001).
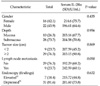
The correlation between the levels of serum-soluble IL-2Rα and the clinicopathologic findings are shown in Table 1. The levels of serum-soluble IL-2Rα in patients with submucosal invasive gastric cancer were significantly than in the patients with mucosal invasive gastric cancer (P = 0.05). We focused on whether or not the serum level of IL-2Rα differed according to lymph node metastasis.
Although patients with lymph node metastasis had higher levels of serum-soluble IL-2Rα than patients without lymph node metastasis, there were no significant differences in the levels of serum-soluble IL-2Rα (257.89 ± 104 in patients with lymph node metastasis [n = 16] and 214.77 ± 95.4 in patients without lymph node metastasis [n = 70]).
The patients were further subdivided according to differentiation of the tumor. In the differentiated type, the serum IL-2Rα level was not useful as a predictor of lymph node metastasis. Based on the ROC curve, the width (0.582) of the differentiated type was significantly less than the undifferentiated type (Fig. 1). Therefore, we limited the study to undifferentiated gastric cancer cases.
In undifferentiated gastric cancer, the levels of serum-soluble IL-2Rα in patients with lymph node metastasis were significantly higher than patients without lymph node metastasis (P = 0.05) (Table 2). The optimal cut-off value of serum-soluble IL-2Rα for predicting lymph node metastasis in patient with early gastric cancer was shown to be 200 U/mL. At this cut-off point, the sensitivity and specificity were 77.8% and 65.5%, respectively (Fig. 2).
Based on this cut-off point, we evaluated the risk factors for lymph node metastasis in patients with undifferentiated gastric cancer (Table 3). As shown in multivariate analysis, a serum IL-2Rα level >200 U/mL was a significant risk factor for lymph node metastasis (P = 0.028).
The development of new treatment modalities, such as EMR, laparoscopic gastrectomy, and robotic surgery, has improved patients' quality of life.
The application of these treatments in patients with early gastric cancer is based on the pre-operative prediction of lymph node metastasis. EMR should not be performed if there is any evidence of lymph node metastasis, even if the tumor is <1 cm in size. In laparoscopic gastrectomy cases, in which lymph node metastasis is determined pre-operatively, the surgeon should consider more than D1 lymph node dissection.
Among the methods of pre-operative evaluation, abdominal CT is the most popular method of evaluation for lymph node metastasis; the most important criterion for lymph node metastasis is the size of the lymph nodes. Generally, a lymph node >10 mm in size is considered to represent tumor metastasis. However, the sensitivity and the specificity for predicting lymph node metastasis of CT scan is unsatisfactory, because 28% of pathologically positive lymph nodes are less than 10 mm in size [9-11]. Another modality for predicting lymph node metastasis in patients with gastric cancer is pre-operative endoscopic ultrasonography, which has the advantage of an accurate diagnostic image of not only the depths of tumor invasion, but also peri-gastric lymph node metastasis.
Use of endoscopic ultrasonography implies that lymph node metastasis can be divided by size and marginal and internal echoes. When lymph node metastasis is present, lymph node echoes are similar to that of stomach lesions, presenting with irregular margins and low echo structures. The diagnosis accuracy of endoscopic ultrasonography is 55 to 75% [12].
In early gastric cancer when the tumor is confined to the mucosa, lymph node metastasis occurs in only 2 to 4% of patients. However, in cases of submucosal invasion, lymph node metastasis occurs in 15 to 25% of patients [13,14]. Lymph node metastasis is the most dangerous risk factor for loco-regional recurrence and distant metastasis.
Abe et al. [15] reported that the high-risk factors for lymph node metastasis include the following: female gender; a tumor invasion of the submucosa; large tumor size; and tumor lymphatic channel invasion. Wu et al. [16] suggested that cases with undifferentiated carcinoma, large tumor size, and submucosal tumor invasion have a high risk for lymph node metastasis. Folli et al. [17] emphasized that endoscopic findings and depth of tumor invasion are important predictors of lymph node metastasis.
The serum IL-2Rα level is a possible alternative or adjuvant tool of diagnosis for pre-operative assessment of lymph node metastasis. Murakami et al. [18] study serum IL-2Rα level can predict lymph node metastasis in early gastric cancer regardless of cell differentiation. However based on the results of our study, only the serum IL-2Rα level in undifferentiated type were different according to lymph node metastasis.
Forones et al. [19] studied 23 patients using IL-2 as a tumor marker associated with careinoembryonal antigen (CEA) and carbohydrate antigen (CA)19-9. In advanced gastric cancer patients, serum levels of IL-2, CEA, and CA19-9 are greatly increased compared to early gastric cancer patients. Maeta et al. [20] found that during the follow-up period, a high level of IL-2 is a poor prognostic factor.
Our study confirmed this result, with gastric cancer patients having an increased serum level of IL-2Rα (222.79 ± 98.1 U/mL) compared to healthy patients (131.96 ± 95.5 U/mL), and the level of serum IL-2Rα was shown to be a meaningful predictor of lymph node metastasis, not in differentiated gastric cancer, but in undifferentiated gastric cancer patients, with a cut-off value of detection of gastric cancer lymph node metastasis based on a serum IL-2 level of 200 U/mL.
A limitation of current study was the small number of cases of lymph node metastasis in undifferentiated group, so statistical power was not as strong as expected. Further studies with a large number of patients are needed.
In conclusion, the serum level of IL-2Rα can be used as a predictor of lymph node metastasis in the patients with undifferentiated early gastric cancer, if it is used in combination with the findings of CT scan and endoscopic ultrasonography.
Figures and Tables
Fig. 1
Receiver operating characteristic (ROC) curve of the differentiated group of early gastric cancers and the undifferentiated gastric cancers.

Fig. 2
Receiver operating characteristic (ROC) curve showing the point of maximal sensitivity and specificity. IL-2Rα, interleukin-2 receptor alpha.

Table 2
Relationship between serum IL-2Rα level and clinicopathologic variables in undifferentiated gastric cancer
References
1. Kim MG, Kim BS, Kim TH, Kim KC, Yook JH, Oh ST, et al. Surgical treatment for patients who underwent endoscopic mucosal resection (EMR)/endoscopic submucosal dissection (ESD) of early gastric cancer (EGC). J Korean Surg Soc. 2011. 80:165–171.
2. Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopyassisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994. 4:146–148.
3. Heo GU, Kim MC, Jung GJ, Choi SR. Robotic gastrectomy for gastric cancer: preliminary results. J Korean Surg Soc. 2009. 76:301–306.
4. Lee JH, Kim JJ. Endoscopic mucosal resection of early gastric cancer: Experiences in Korea. World J Gastroenterol. 2007. 13:3657–3661.
5. Nitti D, Marchet A, Olivieri M, Ambrosi A, Mencarelli R, Farinati F, et al. Lymphadenectomy in patients with gastric cancer. A critical review. Suppl Tumori. 2003. 2:S35–S38.
6. Yanai H, Noguchi T, Mizumachi S, Tokiyama H, Nakamura H, Tada M, et al. A blind comparison of the effectiveness of endoscopic ultrasonography and endoscopy in staging early gastric cancer. Gut. 1999. 44:361–365.
7. Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988. 240:1169–1176.
8. Saito H, Tsujitani S, Katano K, Ikeguchi M, Maeta M, Kaibara N. Levels of serum-soluble receptor for interleukin-2 in patients with colorectal cancer. Surg Today. 1998. 28:1115–1117.
9. Fukuya T, Honda H, Hayashi T, Kaneko K, Tateshi Y, Ro T, et al. Lymph-node metastases: efficacy for detection with helical CT in patients with gastric cancer. Radiology. 1995. 197:705–711.
10. Dehn TC, Reznek RH, Nockler IB, White FE. The pre-operative assessment of advanced gastric cancer by computed tomography. Br J Surg. 1984. 71:413–417.
11. Dorfman RE, Alpern MB, Gross BH, Sandler MA. Upper abdominal lymph nodes: criteria for normal size determined with CT. Radiology. 1991. 180:319–322.
12. Dittler HJ, Siewert JR. Role of endoscopic ultrasonography in gastric carcinoma. Endoscopy. 1993. 25:162–166.
13. Maehara Y, Okuyama T, Oshiro T, Baba H, Anai H, Akazawa K, et al. Early carcinoma of the stomach. Surg Gynecol Obstet. 1993. 177:593–597.
14. Tsujitani S, Oka S, Saito H, Kondo A, Ikeguchi M, Maeta M, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery. 1999. 125:148–154.
15. Abe N, Watanabe T, Suzuki K, Machida H, Toda H, Nakaya Y, et al. Risk factors predictive of lymph node metastasis in depressed early gastric cancer. Am J Surg. 2002. 183:168–172.
16. Wu CY, Chen JT, Chen GH, Yeh HZ. Lymph node metastasis in early gastric cancer: a clinicopathological analysis. Hepatogastroenterology. 2002. 49:1465–1468.
17. Folli S, Morgagni P, Roviello F, De Manzoni G, Marrelli D, Saragoni L, et al. Risk factors for lymph node metastases and their prognostic significance in early gastric cancer (EGC) for the Italian Research Group for Gastric Cancer (IRGGC). Jpn J Clin Oncol. 2001. 31:495–499.
18. Murakami S, Sakata H, Tsuji Y, Okubo K, Hamada S, Hirayama R. Serum soluble interleukin-2 receptor as a predictor of lymph node metastasis in early gastric cancer. Dig Surg. 2002. 19:9–13.
19. Forones NM, Mandowsky SV, Lourenço LG. Serum levels of interleukin-2 and tumor necrosis factor-alpha correlate to tumor progression in gastric cancer. Hepatogastroenterology. 2001. 48:1199–1201.
20. Maeta M, Saito H, Katano K, Kondo A, Tsujitani S, Makino M, et al. A progressive postoperative increase in the serum level of soluble receptors for interleukin-2 is an indicator of a poor prognosis in patients with gastric cancer. Int J Mol Med. 1998. 1:113–116.




 ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download