Abstract
Purpose
Excess weight and obesity have been associated with numerous diseases including thyroid cancer, but the relationship has been weak. The objective of this study was to evaluate the relationship of body sizes on thyroid nodules in healthy Korean population.
Methods
A total of 7,763 persons who underwent a health examination in our health examination center were included in this study. The epidemiologic factors, body size and thyroid ultrasound results were reviewed. We investigated the effects of body size on the presence of thyroid nodules and malignancy.
Results
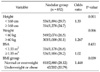
The incidence of thyroid nodules was 20.6%. In the group who were found to have thyroid nodules, mean height, weight and body surface area (BSA) were significantly smaller compared to the others. Especially, in the women, smaller height (less than 160 cm) and overweight (≥ 60 kg) were identified as independent risk factors for the presence of thyroid nodules. The patients with body mass index (BMI) subgroups of normal or overweight had a tendency to have thyroid nodules more frequently. The detection rate of thyroid cancer was 0.47%. The patients with thyroid cancer tended to be smaller in height and BSA than the others.
Excess weight and obesity have been associated with an increased risk of musculoskeletal disorders, hypertension, diabetes, cardiovascular disease, and metabolic syndrome [1]. And they are also associated with a high risk of some common adult cancers, including thyroid cancer [2]. Positi ve associations between height, weight and body mass index (BMI) and thyroid cancer have been reported in large prospective studies and meta-analysis, but these associations generally have been weak and have not always been consistent across studies [3-7]. Most of these studies have looked into the association within Caucasians, and studies on Asian are insufficient. Recently, Suzuki et al. [8] reported the anthropometric factors which had effected on the risk of thyroid cancer. In that study, they showed a positive association between current weights, weight at age 20 years and height, and thyroid cancer risk.
The frequency of obesity has recently been increasing in Korea, although it is not as frequent as with Caucasians. Usually Koreans have the enough iodine nutrition and the incidence of thyroid cancer has been increased sharply in recent, so studies on Koreans are needed. Hence, the authors investigated the effects of height, weight and BMI on thyroid nodules for healthy people.
The subjects of this study were persons who underwent a health examination from January 2009 to December 2009 in health examination in Gyeongsang National University Hospital. Twenty-five patients who had other underlying malignancies and an operation history of the thyroid were excluded from the subjective group.
The epidemiologic factors and body size were determine based on records of health examinations, and BMI and body surface area (BSA) were calculated according to their determined formula (i.e., BMI = weight (kg)/height (m)2, BSA = √weight × height/3600). The BMI results were categorized using the World Health Organization classification; BMI <18.5 benign considered underweight; 18.5 to 24.9, normal; 25 to 29.9, overweight; and >30, obese [9].
All the persons who received health examinations underwent neck ultrasonography, and when a thyroid nodule was discovered, the existence of a fine needle aspiration (FNA) was determined in accordance with the Aemerican Thyroid Association guideline [10].
All data are expressed as mean ± standard deviation. The SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. A P-value less than 0.05 was considered statically significant.
A total of 7,763 persons were studied; 4,681 men, and 3,082 women. The mean age was 46.22 ± 10.63 years, and mean BMI was 23.79 ± 2.94 kg/m2. The overall prevalence of each BMI category was as follows; 2.7% were underweight, and 65.0% were normal, 29.8% were overweight and 2.5% were obese. The number of subjects who were found to have a thyroid nodule was 1,603 (20.6%), and a FNA was performed to 1,402 subjects. Among those who underwent FNA, 37 (2.31%) of those who had a thyroid nodule were found to be malignant, and 1,365 subjects were found to be benign.
In the group who were found to have thyroid nodules, the percentage of women was 53.2%; higher than that of men and the mean age of the patients with thyroid nodule was also higher (P = 0.000). In the women, the mean height, weight and BSA of the group who were found to have thyroid nodules were significantly smaller compared to the others. But there were no statistical differences for the body sizes in the men (Table 1).
In the women, 852 (27.64%) people had thyroid nodules. When we compared the rate of thyroid nodules according to their body sizes (Table 2), the height less than 160 cm and weight more than 60 kg were identified the significant risk factors of thyroid nodules (odds ratio, 1.33 and 1.267). Also the patients who were normal or overweight in BMI subgroup were identified to have higher frequency of thyroid nodules. However, we could not find the significant relationship between body sizes and thyroid nodule in the men.
Among the subjects, malignant cases were 37 (0.47%) of the total (0.28% of men and 0.74% of women). The patients who were concluded with thyroid cancer had significantly smaller mean height and BSA than the others. At the analysis according to sex, however, the height of men was the only significant factor to the existence of malignant nodules of thyroid (Table 3).
Thyroid nodule is one of the most common diseases originating from the endocrine systems. When thyroid nodules discovered through thyroid ultrasonography are included as well as clinically palpable thyroid nodules, the prevalence of thyroid nodules has been reported as 14 to 41% [11-14]. It has been reported that thyroid nodules increase with age and their frequency is higher among women. When neck ultrasonography was performed for healthy persons with no symptoms in this study, the frequency of thyroid nodules was 20.6%, and tended to be higher among women and increase with age. When the correlation between body size and thyroid nodule was examined, however, the group who were found to have thyroid nodules were smaller in height, weight and BSA (P = 0.000). The older persons tended to have smaller height and weight, but the multivariate analysis found that height had statistical significance.
Most thyroid nodules are benign tumors. However, 5 to 15% were reported as malignant, and it is a clinically important task to differentiate them. It has been reported that the frequency of malignancy increases among persons aged under 20 or persons aged 60 or over. An association with a history of radiation exposure has been also found [11-13]. The detection rate of thyroid cancer in health exam was 0.47%, which was very low compared to another studies. So we could not find the significant cut-off values of the body sizes effecting to thyroid cancer.
Thyroid nodule is one of the most common diseases originating from the endocrine systems. Recently many studies on the correlations between thyroid cancer and height, weight, and BMI have been published. A study by Iribarren et al. [14] reported that there were no correlations between thyroid cancer and height, weight, or BMI. Dal Maso et al. [15] reported only a weak correlation between thyroid cancer and height. According to a large-scale study on Norwegians in England, higher BMI or height increased the frequencies of papillary thyroid cancer and follicular cancer among both men and women a little, and the opposite tendency was observed for medullary cancer [3]. Renehan et al. [2] conducted a meta-analysis of five prospective studies on thyroid cancer and BMI, and reported that the risk of thyroid cancer for each increase of 5 in BMI among men and women were 1.52 and 1.14, respectively. According to a study by Sung et al. [16] on the development of various cancers by height, the greater the height, the higher the frequency of thyroid cancer became for both men and women. According to the hypotheses about these correlations, the increase of fat is associated with the increase of insulin which also affects an insulin-like growth factor, and this increases the development of cancer. Another hypothesis postulates that an increase in sex hormones is associated with the increasing development of cancer among women. Yet another hypothesis claims that people with greater height or BMI tend to have larger organs which increase the likelihood of cancerous developments of cells [15-20].
This study has a limitation in that it compared body indexes at a certain point of time depending on the existence of cancer rather than investigating the frequency of cancer through follow-up. According to a study in 2000 which analyzed 12 case-control studies and compared the height, weight and BMI of subjects at the time of diagnosis of thyroid cancer with those of the control group, the group who were diagnosed as having thyroid cancer showed higher indexes among women, but no significant differences were found among men, and there were large differences regarding this between studies [15]. This study also found no statistically significant differences between the benign group and the malignant group who were found from health examination.
Mijović et al. [17] conducted a study on patients with thyroid nodules and indeterminate significance on FNA reported that the higher the BMI was, the lower the malignancy rate was except for women aged 45 or older. Although the subjects are different, the underweight and obese groups in this study showed a higher malignancy rate, although there was no statistical significance.
In this study, the percentage of thyroid nodules among healthy persons discovered from health examination was 20.6%. A higher frequency of thyroid nodules was associated with women, older age. In the women, the height less than 160 cm and the overweight (≥60 kg) were significantly associated with the presence of thyroid nodules. And the patients with normal or overweight of BMI subgroup had tendency more frequent thyroid nodules than others. The detection rate of thyroid cancer was 0.47%. The patients with thyroid cancer were founded to have smaller mean values in height and BSA. But we could not find the significant factors of body sizes which might influence the development of the thyroid cancer. More cases are needed to identify the risk factors of thyroid cancer.
Figures and Tables
References
1. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010. 303:235–241.
2. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008. 371:569–578.
3. Engeland A, Tretli S, Akslen LA, Bjørge T. Body size and thyroid cancer in two million Norwegian men and women. Br J Cancer. 2006. 95:366–370.
4. Goodman MT, Kolonel LN, Wilkens LR. The association of body size, reproductive factors and thyroid cancer. Br J Cancer. 1992. 66:1180–1184.
5. Mack WJ, Preston-Martin S, Bernstein L, Qian D. Lifestyle and other risk factors for thyroid cancer in Los Angeles County females. Ann Epidemiol. 2002. 12:395–401.
6. Paes JE, Hua K, Nagy R, Kloos RT, Jarjoura D, Ringel MD. The relationship between body mass index and thyroid cancer pathology features and outcomes: a clinicopathological cohort study. J Clin Endocrinol Metab. 2010. 95:4244–4250.
7. Ron E, Kleinerman RA, Boice JD Jr, LiVolsi VA, Flannery JT, Fraumeni JF Jr. A population-based case-control study of thyroid cancer. J Natl Cancer Inst. 1987. 79:1–12.
8. Suzuki T, Matsuo K, Hasegawa Y, Hiraki A, Kawase T, Tanaka H, et al. Anthropometric factors at age 20 years and risk of thyroid cancer. Cancer Causes Control. 2008. 19:1233–1242.
9. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000. 894:i–xii. 1–253.
10. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009. 19:1167–1214.
11. Franceschi S, Boyle P, Maisonneuve P, La Vecchia C, Burt AD, Kerr DJ, et al. The epidemiology of thyroid carcinoma. Crit Rev Oncog. 1993. 4:25–52.
12. Mandel SJ. A 64-year-old woman with a thyroid nodule. JAMA. 2004. 292:2632–2642.
13. Hegedüs L. Clinical practice. The thyroid nodule. N Engl J Med. 2004. 351:1764–1771.
14. Iribarren C, Haselkorn T, Tekawa IS, Friedman GD. Cohort study of thyroid cancer in a San Francisco Bay area population. Int J Cancer. 2001. 93:745–750.
15. Dal Maso L, La Vecchia C, Franceschi S, Preston-Martin S, Ron E, Levi F, et al. A pooled analysis of thyroid cancer studies. V. Anthropometric factors. Cancer Causes Control. 2000. 11:137–144.
16. Sung J, Song YM, Lawlor DA, Smith GD, Ebrahim S. Height and site-specific cancer risk: a cohort study of a korean adult population. Am J Epidemiol. 2009. 170:53–64.
17. Mijović T, How J, Pakdaman M, Rochon L, Gologan O, Hier MP, et al. Body mass index in the evaluation of thyroid cancer risk. Thyroid. 2009. 19:467–472.
18. Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004. 23:6365–6378.
19. Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002. 3:565–574.
20. Leitzmann MF, Brenner A, Moore SC, Koebnick C, Park Y, Hollenbeck A, et al. Prospective study of body mass index, physical activity and thyroid cancer. Int J Cancer. 2010. 126:2947–2956.




 ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download