Abstract
A small-cell carcinoma is one of the histologic subtypes of primary neuroendocrine carcinomas of the breast. A small-cell carcinoma is a rare entity of the breast and exhibits similar morphologic features as neuroendocrine tumors of the gastrointestinal tract and lung. We present the imaging and pathologic findings of a primary small-cell neuroendocrine carcinoma of the breast. This is the first report of a primary small-cell carcinoma arising from the breast in Korea.
Neuroendocrine neoplasms are defined as epithelial neoplasms with predominant neuroendocrine differentiation, which range from non-aggressive carcinoid tumors to highly aggressive small cell carcinomas [1]. Neuroendocrine neoplasms arise in most organs of the body, especially the lungs, pancreas, and gastrointestinal tract [2]. Since Wade et al. [3] first reported a neuroendocrine tumor in 1983, several cases have been reported. We present a case of a primary neuroendocrine carcinoma of the breast with imaging and pathologic findings.
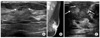
In July 2007, a 64-year-old woman sought evaluation at our hospital for a palpable lesion in the left breast which had been detected several days earlier. The medical history was significant for diabetes mellitus and hypertension. A 0.8-cm irregular, hypoechoic lesion near the left areola was demonstrated on sonography (Fig. 1A). A biopsy was recommended due to the suspicious shape; however, the patient declined any procedures which would permit pathologic confirmation.
In December 2010, the patient visited our hospital with symptoms of exertional dyspnea of 1 month duration. Because of the underlying diabetes mellitus and hypertension, the imaging work-up focused on the pulmonary symptoms. A chest computed tomography (CT) showed multiple nodular septa, fissures, and pleural thickening of both lungs with a large bilateral pleural effusion and multiple enlarged lymph nodes in the paratracheal and axillary areas, suggestive of lymphangitic metastasis. There were no pulmonary nodules, mediastinal masses, or bronchial abnormalities. In addition to the pulmonary findings, a large irregular mass was incidentally detected in the left breast with overlying skin thickening and nipple retraction. The breast mass was evaluated by mammography and sonography. On mammography, an ill-defined hyperdense mass occupied the left subareolar area which was adherent to the areola (Fig. 1B). Nipple retraction, diffuse skin thickening, and multiple enlarged lymph nodes in the left axilla were noted with shrinkage of the volume of the left breast. An irregular hypoechoic mass with invasion to the nipple was demonstrated on sonography (Fig. 1C). Abdominal and pelvic CT and positron emission tomography-CT were obtained to check the other organs of the body, and showed no evidence of abdominal or pelvic lesions. The final diagnosis of the imaging studies was a primary breast tumor with pulmonary metastasis. We took a biopsy of the breast mass to confirm the pathology. Microscopic finding showed infiltrating nests of small cells in the fibrotic stroma, the tumor cells of which had small hyperchromatic nuclei and scanty cytoplasms (Fig. 2A). Immunohistochemical stain showed strong positivity of the tumor cells for neuron-specific enolase, suggestive of small cell neuroendocrine carcinoma (Fig. 2B).
Primary neuroendocrine carcinomas of the breast exhibit morphologic features similar to neuroendocrine tumors of the gastrointestinal tract and lungs. Primary neuroendocrine carcinomas express neuroendocrine markers in >50% of the cell population. Neuroendocrine carcinomas of the breast represent 2 to 5% of breast carcinomas. In 2003, the World Health Organization classification of tumors included three histologic subtypes of neuroendocrine tumors: solid neuroendocrine carcinoma; small cell carcinoma; and large cell neuroendocrine carcinoma. A small cell neuroendocrine carcinoma is morphologically indistinguishable from its counterpart in the lung on the bases of histologic and immunohistochemical features [4]. Therefore, exclusion of an extramammary primary site and/or demonstration of an in situ component within the breast should be confirmed to diagnose the primary small cell neuroendocrine carcinoma of the breast [5]. Primary small cell neuroendocrine carcinomas of the breast as also known as oat cell carcinomas, high-grade neuroendocrine carcinomas, or small cell undifferentiated carcinomas.
The histologic characteristics of primary small cell neuroendocrine carcinomas are scant cytoplasm, fine granular chromatin, inconspicuous nucleoli, and a high mitotic rate. Necrosis is common and may be associated with other conventional breast carcinoma patterns. There is no consistent pattern of neuroendocrine marker expression; however, most tumors are reactive for neuron-specific enolase, cytokeratin, and one or more neruoendocrine differentiation indicators (Grimelius stain, synaptophysin, Leu 7, serotonin, bombesin, and chromogranin A or B) [6]. Estrogen and progesterone receptors are expressed in >50% of small cell carcinomas [4]. The histogenesis of primary small cell neuroendocrine carcinomas is still unclear. It has been suggested that a small cell neuroendocrine carcinoma is a variant of a metaplastic carcinoma arising from usual lobular or ductal carcinoma [4] or multipotential stem cells capable of divergent differentiation [7].
Breast small cell carcinomas should be differentiated from small cell carcinomas from another site, lobular carcinomas, or lymphomas; immunohistochemistry may help to distinguish such lesions. Breast small cell carcinomas are positive for cytokeratin 7 and negative for cytokeratin 20, whereas pulmonary small cell carcinomas are negative for both markers [4]. E-cadherin is negative in lobular carcinomas, but 100% positive in small cell carcinomas [8]. Lymphomas are immunoreactive for leukocyte common antigen, CD20, or CD3. In the current case, light microscopy showed infiltrating small discohesive cells in the hyalinized stroma. The tumor cells had small nuclei and scanty cytoplasm admixed with reactive small lymphocytes. The tumor cells were initially thought to be lymphoid cells, but immunohistochemical stains revealed positive for epithelial membrane antigen and cytokeratin, but negative for leukocyte common antigen, CD3, CD20. The tumor cells were positive for neuron-specific enolase and E-cadherin. Thus, we excluded lymphomas and lobular carcinomas from the differential diagnosis. Perineural invasion and tumor necrosis were present. Estrogen and progesterone receptors were negative, Her-2 was three positive, and Ki-67 was elevated (70%), suggesting aggressive tumor characteristics.
The serum tumor marker CA15-3 was within normal limit in our case and was not helpful to differentiate the primary lesion. Kinoshita et al. [7] reported elevated levels of serum carcinoembryonic antigen and NCC-ST-439 and Kitakata et al. [9] reported that the levels of the serum tumor markers carcinoembryonic antigen, CA15-3, NCC-ST-439, and squamous cell carcinoma antigen were within normal limits. More data seem to be necessary to find a constant pattern of the serum tumor markers about the primary small cell carcinomas of the breast.
Small cell neuroendocrine carcinomas of the breast have been reported in several cases, which is not sufficient to characterize the imaging findings of the tumor [9,10]. The radiologic findings of our case were similar to advanced breast malignancy of the other histologic types. Although the pathologic findings were not confirmed, we postulated that the palpable small lesion detected in 2007 might have been an early stage of this mass. The small lesion was located at the 1 o'clock direction near the left nipple in 2007 and the imaging studies obtained in 2010 showed that the mass was in the same location. In such a case, it is likely that it took 3 years for the early lesion to progress to an advanced lesion. Although the period during which malignant lesions progress is variable and depends on the histologic type, grade, and patient's health, the duration of time could be a feature of progression of small cell neuroendocrine carcinomas of the breast. Small cell carcinomas have been considered as undifferentiated carcinomas and aggressive tumors, resulting in poor prognosis. Recently, it has been reported that the prognosis depends on the stage at the time of diagnosis and that low-stage small cell carcinomas respond to conventional treatment without progression of the disease [9].
In summary, the primary small cell carcinoma of the breast in our patient presented with imaging findings similar to the usual type of ductal carcinoma in early and advanced stages. Although the period of tumor progression depends on variable factors, our case could serve as a valuable example to assess the biology of this tumor. Immunohistochemistry may help to differentiate this rare breast malignancy from the other tumors which have similar gross and microscopic features.
Figures and Tables
Fig. 1
(A) Sonography showed small irregular hypoechoic lesion with angular margin and spiculations (arrows). (B) Mammography showed ill-defined hyperdense mass in left subareolar area which was adherent to areola. Nipple retraction, diffuse skin thickening, and multiple enlarged lymph nodes in left axilla were noted with shrinkage of volume of left breast. (C) Sonography showed irregular hypoechoic mass with invasion to nipple (arrows).
References
1. Sapino A, Bussolati G. Is detection of endocrine cells in breast adenocarcinoma of diagnostic and clinical significance? Histopathology. 2002. 40:211–214.
2. Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008. 9:61–72.
3. Wade PM Jr, Mills SE, Read M, Cloud W, Lambert MJ 3rd, Smith RE. Small cell neuroendocrine (oat cell) carcinoma of the breast. Cancer. 1983. 52:121–125.
4. Shin SJ, DeLellis RA, Ying L, Rosen PP. Small cell carcinoma of the breast: a clinicopathologic and immunohistochemical study of nine patients. Am J Surg Pathol. 2000. 24:1231–1238.
5. Rosen PP. Rosen PP, editor. Mammary carcinoma with endocrine features. Rosen's breast pathology. 2001. 2nd ed. Philadelphia: Lippincott Williams & Wilkins;503–508.
6. Papotti M, Gherardi G, Eusebi V, Pagani A, Bussolati G. Primary oat cell (neuroendocrine) carcinoma of the breast. Report of four cases. Virchows Arch A Pathol Anat Histopathol. 1992. 420:103–108.
7. Kinoshita S, Hirano A, Komine K, Kobayashi S, Kyoda S, Takeyama H, et al. Primary small-cell neuroendocrine carcinoma of the breast: report of a case. Surg Today. 2008. 38:734–738.
8. Shin SJ, DeLellis RA, Rosen PP. Small cell carcinoma of the breast--additional immunohistochemical studies. Am J Surg Pathol. 2001. 25:831–832.
9. Kitakata H, Yasumoto K, Sudo Y, Minato H, Takahashi Y. A case of primary small cell carcinoma of the breast. Breast Cancer. 2007. 14:414–419.
10. Mariscal A, Balliu E, Díaz R, Casas JD, Gallart AM. Primary oat cell carcinoma of the breast: imaging features. AJR Am J Roentgenol. 2004. 183:1169–1171.




 ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download