Abstract
Chylous ascites is defined as the accumulation of chyle in the peritoneum due to obstruction or rupture of the peritoneal or retroperitoneal lymphatic glands. Chylous ascites that arises from acute pancreatitis with portal vein thrombosis is very rare. We report here on a case of chylous ascite that was caused by acute pancreatitis with portal vein thrombosis, in which the patient showed an impressive response to conservative therapy with total parenteral nutrition and octerotide. We also review the relevant literature about chylous ascites with particular reference to the management of this rare disease.
Since Morton's dramatic and detailed account in 1694 of a 2-year-old boy who died with chylous ascites (caused by tuberculosis), the accumulation of chylous fluid in the peritoneal cavity is known to be a rare condition [1]. The majority of cases in adults are attributed to malignancies, while congenital lymphatic abnormalities are involved in the pediatric population. Most of the patients with chylous ascites are known to have a poor outcome because of the high proportion of malignancy being the cause of chyloperitoneum. We concluded that acute pancreatitis with portal vein thrombosis was the cause of chylous ascites in this currently reported case. In this case, an inflammatory process of the pancreatitis could have initiated structural and functional damage to the main lymphatic tissue; and the portal hypertension due to portal vein thrombosis served as an aggregating factor to increase the chylous ascites. We report here on an uncommon case of chylous ascites following acute pancreatitis with portal vein thrombosis in which the patient responded to only parenteral nutrition and octreotide.
A 63-year-old man visited our hospital for his increasing abdominal distension during the previous 2 months. For his medical history, he received operative management for meningioma of the brain. He was diagnosed as having portal vein thrombosis due to acute pancreatitis in April 2009 (Fig. 1), and since then had taken medication (warfarin) for 1-year. He had no risk factors for chronic liver disease. After the brain operation, he has been bed ridden for over half a day, daily, due to motor weakness after the surgical management of meningioma. He experienced weight loss of 4 kg during the next 6 months and experienced persistent anorexia. On physical examination, we did not detected any specific findings except for shifting dullness within a distended abdomen.
For the laboratory studies, the hemoglobin level, white blood cell and platelet count was 11.6 g/uL (normal range, 13 to 18), 4.66 × 103/uL (normal range, 4 to 10), and 137 × 103/uL (normal range, 150 to 450), respectively. The liver function tests disclosed an aspartate aminotransferase level of 30 IU/L (normal range, 5 to 35), an alanine aminotransferase level of 20 IU/L (normal range, 5 to 40), an alkaline phosphatase level of 344 IU/L (normal range, 104 to 338), a total bilirubin level and direct bilirubin level of 0.67 mg/dL (normal range, 0.2 to 1.4) and 0.25 mg/dL (normal range, 0 to 0.7), respectively; an albumin level of 3.8 g/dL (normal range, 3.8 to 5.1), an amylase level of 155 IU/L (normal range, 28 to 100) and a lipase level of 47 IU/L (normal range, 13 to 60). We did not find any factors that could give rise to portal vein thrombosis.
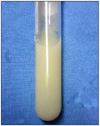
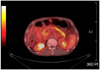
Abdominal computed tomography (CT) revealed the portal vein thrombosis from the intrahepatic portal vein to the proximal part of the superior mesenteric vein and there was a cavernous transformation of the portal vein with a patent hepatic vein and splenic vein. A large amount of ascites in the perihepatic, subphrenic, paracolic and pelvic spaces were present. There was also edematous change of the pancreas head and body without lymphadenopathy or a fluid collection (Fig. 2). Paracentesis drained some of the milky whitish color fluid with a high triglyceride level of 57 mg/L and an albumin level of 1.3 g/L, which is consistent with chylous ascites (Fig. 3). On the cell count analysis of the ascitic fluid, the red blood cells were 920/µL and the white blood cells were 150µL (polymorphous nuclear cells, 10% and mononuclear cells, 90%). The chylous ascites caused by infection could be excluded because acid fast bacilli stain and culture, gram stain and culture, fungal culture and polymerase chain reaction for tuberculosis were all negative. The serum α-fetoprotein and carcinoembryonic antigen were within the normal ranges. The serum CA19-9 levels were raised to 279 IU/mL (0 to 33). We performed flourodeoxyglucose-positron emission tomography-CT (FDG-PET-CT) to exclude internal malignancy. PET-CT showed the hot uptake of radioactive agent in only the entire pancreas without abnormal uptake of the peritoneal organs and lymph nodes (Fig. 4). The 5-hydroxyindolacetic acid titer was also unremarkable. The histologic examination of the chylous ascites also showed reactive change without malignant cells or dysplastic cells. Immunohistologic testing of the patient's serum was performed to exclude autoimmune pancreatitis. Serum immunoglobulin (Ig) G and IgG subclass 4 was 1,697 mg/dL (range, 700 to 1,600) and 527.0 mg/dL, respectively. Anti-nuclear antibody and anti-mitochondrial antibody were negative. Esophagogastroduodenoscopy showed esophageal varix of grade I to II. Lymphoscintigraphy was not performed. We concluded that the chylous ascites was caused by portal vein thrombosis with acute pancreatitis. He was treated with diuretics, total parenteral nutrition and octreotide. After 1 week, he showed improvement of distended abdomen and this management option was maintained for three weeks with a lower fatty diet with median chain triglycerides (MCT) supplementation. Since then, he was continuously treated with a lower fatty diet with MCT and diuretics. The decrease in the chylous ascites was confirmed by follow-up CT scan. During follow-up every 3 months, the CA19-9 level of the serum was increased to more than 1,000 IU/mL without an increase of the chylous ascites. We re-evaluated the patient to find evidence of internal malignancy; except for a high CA19-9 level, we could not find evidence of malignancy.
Chylous ascites is a rare form of ascites and this is characterized by a milky fluid that contains a high level of triglycerides. The diagnosis of chylous ascites requires peritoneal fluid with at least one of the following criteria [2]: peritoneal lipid content greater than that of plasma, a peritoneal to plasma protein concentration ratio of >0.5, a triglyceride concentration >110 mg/dL, and microscopic fat. The incidence of chylous ascites is from 1 in 20,000 to 1 in 187,000 admissions at large tertiary referral hospitals [3].
The mechanisms for the formation of chylous ascites are related to the following: 1) primary lymph node invasion by malignancy obstructing lymph flow, resulting in leakage of lymph from the subserosal lymphatics into the peritoneal cavity, 2) exudation of lymph through congenital retroperitoneal megalymphatics, 3) obstruction of the thoracic duct by trauma resulting in leakage of chyle, and 4) increasing caval or hepatic pressure from congestive conditions such as right side heart failure and liver cirrhosis [4].
The causes of chylous ascites can roughly be divided into traumatic and atraumatic. For traumatic causes, surgical procedure and therapeutic intervention are well known causes of chylous ascites. Especially, surgeries that require dissecting lymphatic tissues such as oncological surgeries are known to frequently cause chylous ascites. For patients undergoing abdominal or retroperitoneal surgery, the incidence of postoperative chylous ascites was reported to be from 1.1 to 7.4% [5]. An additional cause of indirect injury leading to the development of chylous ascites is radiation. The abdominal radiation that causes chylous ascites is thought to induce fibrosis of the lymphatic vessels within the small bowel and the mesentery; this causes obstruction and subsequent extravasation of chylous [6]. The finding of atraumatic chylous ascites should always prompt the surgeon to rule out these serious underlying causes. In the pediatric population, the predominant cause of chylous ascites is known to be lymphatic anomalies in up to 84% of the cases [7]. In contrast, malignancies, liver cirrhosis, and mycobacterium infections are known as the major causes in the adult population. Lymphatic anomalies account for only for 9% of the cases of atraumatic chylous ascites in adults [8].
According to a review of the literature, 13 cases have been reported during the last several decades: there were seven cases of chylous ascites associated with chronic pancreatitis, one case associated with chronic relapsing pancreatitis and only five cases associated with acute pancreatitis [8]. Our case showed different clinical findings when compared with those of the previously reported cases. First, our case had portal vein thrombosis with cavernous change of the portal vein before the formation of chylous ascites. This finding and the acute pancreatitis finding that was shown in 18 FDG-PET-CT had an influence on the development of the chylous ascites, although this case did not show the typical clinical findings and laboratory findings of acute pancreatitis. Second, we cannot exclude pancreatic cancer or internal malignancy because of the continuously high CA19-9 level of the serum. At this point it is difficult to say for sure what caused the chylous ascites in this patient.
Different therapeutic options have been described for the management of chylous ascites, including surgery, octreotide and a low fat diet with medium chain triglyceride supplementation with parenteral nutrition [6,9]. Aalami et al. [6] were retrospectively reviewed in case reports according to therapeutic option. The patients who were included had chylous ascites from multiple causes that resolved after either medical or surgical intervention. One fifth of the total 156 patients was treated surgically; 105 patients were treated conservatively. The patients were first treated with conservatively, followed-up with total parenteral nutrition, high protein with low fat diet, and paracentesis. Octreotide also is available for control of chylous ascites. Octreotide decreases splanchnic and portal blood flow, portal pressure and postprandial hyperemia response in portal hypertensive patients without having significant effects upon the systemic circulation [10]. Surgical exploration is recommended for chylous ascites with a benign histological cause that has been refractory to an appropriative trial of vigorous nonoperative treatment. Leong et al. [10] first reported the use of octreotide in chylous ascites caused by portal hypertension in portal vein thrombosis. Of all the patients, 67% were successfully treated conservatively, and 33% were successfully treated surgically [6]. Our patient showed a good response with a decreasing amount of chylous ascites within a few weeks after being treated with parenteral nutrition, octreotide injection and administering diuretics.
In spite of the high serum CA19-9 levels, our patient is alive without having developed chylous ascites or internal malignancy during 2 years of follow-up.
We have reported here on an unusual case of chylous ascites that was caused by acute pancreatitis with portal hypertension that improved by administering total parenteral nutrition with the patient in a fasting state, octreotide injection and diuretics.
Figures and Tables
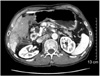 | Fig. 1Computed tomography scan showing portal vein thrombosis with cavernous change of portal vein before chylous ascites occurred. |
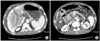 | Fig. 2Computed tomography scan showing large amount of ascites with portal vein thrombosis and head of pancreas showed edematous enlargement with mild pancreatic duct dilatation. |
References
1. Malagelada JR, Iber FL, Linscheer WG. Origin of fat in chylous ascites of patients with liver cirrhosis. Gastroenterology. 1974. 67:878–886.
2. Cardenas A, Chopra S. Chylous ascites. Am J Gastroenterol. 2002. 97:1896–1900.
3. Almakdisi T, Massoud S, Makdisi G. Lymphomas and chylous ascites: review of the literature. Oncologist. 2005. 10:632–635.
4. Kaas R, Rustman LD, Zoetmulder FA. Chylous ascites after oncological abdominal surgery: incidence and treatment. Eur J Surg Oncol. 2001. 27:187–189.
5. Browse NL, Wilson NM, Russo F, al-Hassan H, Allen DR. Aetiology and treatment of chylous ascites. Br J Surg. 1992. 79:1145–1150.
6. Aalami OO, Allen DB, Organ CH Jr. Chylous ascites: a collective review. Surgery. 2000. 128:761–778.
7. Vignes S, Bellanger J. Primary intestinal lymphangiectasia (Waldmann's disease). Orphanet J Rare Dis. 2008. 3:5.
8. Steinemann DC, Dindo D, Clavien PA, Nocito A. Atraumatic chylous ascites: systematic review on symptoms and causes. J Am Coll Surg. 2011. 212:899–905.
9. Leibovitch I, Mor Y, Golomb J, Ramon J. The diagnosis and management of postoperative chylous ascites. J Urol. 2002. 167(2 Pt 1):449–457.
10. Leong RW, House AK, Jeffrey GP. Chylous ascites caused by portal vein thrombosis treated with octreotide. J Gastroenterol Hepatol. 2003. 18:1211–1213.




 Citation
Citation Print
Print


 XML Download
XML Download