Abstract
Inflammatory myofibroblastic tumor (IMT) of the biliary tree is extremely rare and is generally a benign condition, though malignant change is possible. Making a differential diagnosis between this lesion and other malignant conditions is very difficult on preoperative imaging studies. Hence, the final diagnosis of IMT may be made during or after operation depending on the pathologic examination. We treated a 63-year-old woman who received right hepatectomy with caudate lobectomy under the suspicion of hilar cholangiocarcinoma. Frozen biopsy during the operation showed carcinoma in situ and there were stromal cells in the bile duct's resection margins. The postoperative hospital course was uneventful except for minor bile leakage. At postoperative month 4, she developed jaundice, ascites and pleural effusion. Computed tomography images showed a mass-like lesion in the porta hepatis with portal vein thrombosis and a right chest wall mass. Excisional biopsy was done and the pathology report was malignant spindle cell tumor suggestive of an aggressive form of IMT. Her condition rapidly deteriorated regardless of the best supportive care and she expired at postoperative month 5. Further investigation is necessary to clarify the reasons for recurrence and infiltration of this disease.
Inflammatory myofibroblastic tumors (IMT) are rare lesions with the gross appearance of a malignancy, but they are generally benign lesions composed of myofibroblastic spindle cells, plasma cells, lymphocytes and eosinophils [1]. This tumor was previously called plasma cell granuloma, inflammatory pseudotumor and inflammatory myofibrohistiocytic proliferation, but IMT is the currently used designation [2]. Tumors located at the hilar region and that are complicated with obstructive jaundice are mostly malignant hilar cholangiocarcinoma and these tumors require surgical resection if possible. The clinical manifestations and radiologic images of IMT are very similar to those of a malignant lesion of the hilar biliary tract. So diagnosis of these lesions usually depends on the pathology during or after an operation. Making a differential diagnosis of benign and malignant tumors is not very difficult, but the final diagnosis of IMT may be somewhat challenging.
We report here on a patient with aggressive IMT located at the porta hepatis along with cholangiocarcinoma in situ of the proximal bile duct.
A 63-year-old woman was admitted to the Department of Surgery at Kyung Hee University Hospital for the evaluation of jaundice and weight loss (5 kg) during the previous 2 months. She had no relevant medical history. The initial liver function tests, including aspartate aminotransferase, alanine aminotransferase, total bilirubin, γ-glutamyltransferase and alkaline phosphatase, were 98 (IU/L), 290 (IU/L), 4.9 (mg/dL), 374 (IU/L) and 838 (IU/L), respectively.
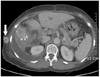
Abdominal ultrasound examination showed dilatation of both the intrahepatic bile ducts. Abdominal computed tomography (CT) examination showed an enhancing mass at the confluent level with dilatation of both intrahepatic bile ducts, and this was all suggestive of Klatskin tumor type IIIa or IV (Fig. 1).
Magnetic resonance cholangiopancreatography also showed dilatation and separation of both intrahepatic bile ducts, as well as suspicion of right hepatic artery encasement by the tumor (Fig. 2).
Endoscopic retrograde cholangiopancreatography was performed to evaluate the biliary tree and decompress the jaundice. There was a stricture at the bifurcation of the hepatic duct, which was suggestive of a cholangiocarcinoma of the common hepatic duct at the porta hepatis (a Klatskin tumor, Bismuth type IIIa). The area of stricture was brushed for cytology and was found to be negative. The guide wire could not be advanced into the right hepatic duct, so an endoscopic nasobiliary drainage tube was placed into the left hepatic duct (Fig. 3). Percutaneous transhepatic biliary drainage was performed for right hepatic duct biliary drainage. Positron emission tomography (PET) was done for evaluation of the lesion and to rule out metastasis. However, there was no visible abnormal hypermetabolic lesion in the porta hepatis.
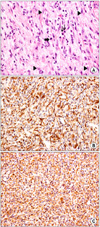
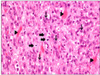
The patient subsequently underwent right hepatectomy with caudate lobectomy and Roux-en-Y hepaticojejunostomy due to the high probability of hilar cholangiocarcinoma. The frozen biopsies of all the bile duct resection margins were negative existing stromal cells. The final pathologic diagnosis was carcinoma in situ of the bile duct with exuberant stromal fibrosis. Immunoreactivity for smooth muscle actin and vimentin was shown (Fig. 4). The patient was diagnosed with inflammatory myofibroblastic tumor with hilar bile duct carcinoma in situ. The hospital course was uneventful except for minor bile leakage and the patient was discharged without complication.
At 4 months after the initial operation, the patient was re-admitted for cholangitis, abdominal distension, dyspnea and a right chest wall mass. Abdominal CT showed a mass-like lesion in the porta hepatis with portal vein thrombosis, intrahepatic bile duct dilatation and a large amount of right pleural effusion with ascites. A right chest wall enhancing mass was detected suggestive of metastatic lesion (Fig. 5).
Excisional biopsy was performed for the chest wall mass, the pathology of which was malignant spindle cell tumor suggestive of the aggressive form of inflammatory myofibroblastic tumor (Fig. 6). Immunohistochemical staining for smooth muscle actin and vimentin was positive as such immunohistochemical staining of the bile duct.
The patient's condition rapidly worsened regardless of supportive care and she expired during the 5th postoperative month.
IMT has been more frequently described in the lung and abdomen, but can also be found in the central nervous system, salivary glands, larynx, bladder, breast, spleen, skin and liver [2]. The pathogenesis of IMT is controversial. Some previous cases were related to autoimmune disease and infectious disease [3,4]. IMTs at the biliopancreatic region, and especially at the distal bile duct, are usually associated with autoimmune pancreatitis, which is believed to be an initial activation process, and this indicates that the fibroblastic cells in both lesions may have been induced at the same site resulting from the same inflammatory and fibrosing process with different histological appearances [3].
Whether this current case belongs to a form of cholangitis associated with lymphoplasmacytic sclerosing pancreatitis is unknown.
IMT of the bile duct is extremely rare with only limited cases having been reported in the literature [5]. The clinical manifestations of hilar biliary duct IMT are indistinguishable from those of hilar cholangiocarcinoma. Radiologic studies of hilar bilary IMT can not provide differentiation between IMT and cholangiocarcinoma or lymphoma due to considerable similarities [6]. In this case, the only difference in cholangiocarcinoma was that there was no visible abnormal hypermetabolic lesion seen on PET.
Most IMTs behave as benign lesions. There is no consensus on the appropriate treatment of IMT. However, surgical resection is the one of the preferred treatments, if possible. But medical treatments such as chemotherapy, radiation therapy, antitumor necrosis factor-α binding antibody, nonsteroidal anti-inflammatory drugs or corticosteroids have been reported [2,7]. A very limited number of cases, as in this case, have demonstrated aggressive features with a significant recurrence rate of 25%. Recurrence is associated with an abdominopelvic site, multinodular tumor masses and incomplete resection, but even IMTs that are only biopsied or excised with positive margins sometimes do not recur or progress [8].
IMT is currently regarded as a locally recurrent, rarely metastasizing neoplasm of intermediate malignancy [2]. There is a report about its different biological behavior that may represent a premalignant lesion [9]. In this case, we are not sure whether the carcinoma in situ of the bile duct was an incidental finding or if it was associated with the pathogenesis of the biliary IMT.
Microscopically, IMT consists of varying proportions of spindle cells, plasma cells, lymphocytes, eosinophils and macrophages. The spindle cells usually stain positively for actin and vimentin, but are negatively stained for S100. This case also stained positive for smooth muscle actin and vimentin, and negative for S100. Anaplastic lymphoma kinase (ALK) immunohistochemistry is relatively specific for IMT among the spectrum of fibroblastic-myofibroblastic tumors and other potential mesenchymal mimics of IMT [10]. The previous study indicated that ALK positive IMT was diagnosed in younger age patients and that it had an improved prognosis. ALK-negative IMTs were associated with a higher age, death due to local disease, and the presence of metastases [2]. Our current case stained negatively for ALK protein.
In conclusion, biliary IMT is extremely rare, and can be one of the causes of obstructive jaundice. Clinicians must remain aware of the aggressiveness of this disease and should keep an eye on the possibility of disease recurrence. Further investigation is necessary in order to better clarify the characteristics and treatment of IMT.
Figures and Tables
Fig. 1
Computed tomograpy shows enhancing mass at confluent level with dilatation of both intrahepatic bile ducts dilatation of intrahepatic duct.
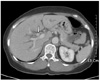
Fig. 3
Endoscopic retrograde cholangiopancreatography findings. Stricture at bifurcation of hepatic duct.
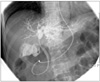
Fig. 4
(A) Inflammatory myofibroblastic tumor of liver composed of atypical spindle cells (arrow heads) with intervening collagen bundles. Mitotoic figure (thick arrow) and plasma cell infiltration (thin arrows) are noted (H&E, ×400). (B) Inflammatory myofibroblastic tumor shows immunoreactivity for smooth muscle actin (polymer method, ×200). (C) Inflammatory myofibroblatic tumor shows immunoreactivity for vimentin (polymer method, ×200).
References
1. Zamir D, Jarchowsky J, Singer C, Abumoch S, Groisman G, Ammar M, et al. Inflammatory pseudotumor of the liver--a rare entity and a diagnostic challenge. Am J Gastroenterol. 1998. 93:1538–1540.
2. Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007. 31:509–520.
3. Martín Malagón A, López-Tomassetti Fernández E, Arteaga González I, Carrillo Pallarés A, Díaz Luis H. Inflammatory myofibroblastic tumor of the distal bile duct associated with lymphoplasmacytic sclerosing pancreatitis. Case report and review of the literature. Pancreatology. 2006. 6:145–154.
4. Rosenbaum L, Fekrazad MH, Rabinowitz I, Vasef MA. Epstein-Barr virus-associated inflammatory pseudotumor of the spleen: report of two cases and review of the literature. J Hematop. 2009. 2:127–131.
5. Ashcroft MW, Ng CS, Frost RA, Freeman AH. Biliary inflammatory pseudotumour: report of two cases and review of the literature. Clin Radiol. 2009. 64:449–455.
6. Venkataraman S, Semelka RC, Braga L, Danet IM, Woosley JT. Inflammatory myofibroblastic tumor of the hepatobiliary system: report of MR imaging appearance in four patients. Radiology. 2003. 227:758–763.
7. Dishop MK, Warner BW, Dehner LP, Kriss VM, Greenwood MF, Geil JD, et al. Successful treatment of inflammatory myofibroblastic tumor with malignant transformation by surgical resection and chemotherapy. J Pediatr Hematol Oncol. 2003. 25:153–158.
8. Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995. 19:859–872.
9. Donner LR, Trompler RA, White RR 4th. Progression of inflammatory myofibroblastic tumor (inflammatory pseudotumor) of soft tissue into sarcoma after several recurrences. Hum Pathol. 1996. 27:1095–1098.
10. Cook JR, Dehner LP, Collins MH, Ma Z, Morris SW, Coffin CM, et al. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study. Am J Surg Pathol. 2001. 25:1364–1371.




 Citation
Citation Print
Print


 XML Download
XML Download