Abstract
TS-1 is an oral anti-cancer agent for gastric cancer with a high response rate and low toxicity. We report a case of long-term drug retention of TS-1 causing interstitial lung disease (ILD) as a fatal adverse reaction. A 65-year-old woman underwent a total gastrectomy with pathologic confirmation of gastric adenocarcinoma. She received 6 cycles of TS-1 and low-dose cisplatin for post-operative adjuvant chemotherapy followed by single-agent maintenance therapy with TS-1. After 8 months, the patient complained of a productive cough with sputum and mild dyspnea. A pulmonary evaluation revealed diffuse ILD in the lung fields, bilaterally. In spite of discontinuing chemotherapy and the administration of corticosteroids, the pulmonary symptoms did not improve, and the patient died of pulmonary failure. TS-1-induced ILD can be caused by long-term drug retention that alters the lung parenchyma irreversibly, the outcome of which can be life-threatening. Pulmonary evaluation for early detection of disease is recommended.
Gastric cancer remains one of the leading causes of cancer deaths worldwide. Complete surgical resection provides the only curative chance for gastric cancer. In spite of recent improvements in overall survival rates, the mortality of patients remains high because many patients are diagnosed in the advanced stages of gastric cancer [1]. In spite of many effective chemotherapeutic agents and various administration methods, it has been shown that gastric cancer is one of the malignant diseases that is less sensitive to chemotherapy [2].
TS-1 is a novel, oral fluorouracil formation composed of tegafur, 5-chloro-2, 4-dihydroxypyridine (CDHP), and potassium oxonate (Oxo) in a molar ratio 1:0.4:1, based on biochemical modulation of 5-fluorouracil [3]. Late phase II clinical trials have already been conducted in gastric cancer patients with a response rate of 44.6%. On the strength of such encouraging results, gastric cancer as an indication for the use of TS-1 was approved in March 1999. As TS-1 is effective when administered orally and has a low incidence of severe adverse effects, TS-1 is expected to be suitable for outpatient chemotherapy [4].
We report the first case of long-term drug retention of TS-1 with a fatal adverse reaction causing interstitial lung disease (ILD).
A 65-year-old woman visited our hospital on 29 April 2008 for evaluation of intermittent epigastric pain which had developed 6 months earlier. The medical history was unremarkable. She has no past history of pulmonary disease and the chest X-ray was normal. Endoscopic evaluation demonstrated a deep ulcerative lesion in the angle to the high body of the stomach; the biopsy revealed it as a poorly differentiated adenocarcinoma. An abdominopelvic computed tomography (CT) scan revealed a thickening of the gastric wall, adjacent fat infiltration, and multiple metastatic lymph nodes around the left gastric artery, suggesting advanced gastric cancer.
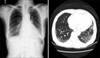
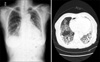
The patient underwent total gastrectomy and splenectomy with standard D2 lymph node dissection on 15 May 2008. Macro- and microscopically, no residual tumor was remained (R0 resection). The pathologic results confirmed a stage IV (T3N3) gastric cancer according to the 6th edition of the American Joint Committee on Cancer/International Union Against Cancer tumor node metastasis classification. She was classified as an Eastern Cooperative Oncology Group performance status of 1 [5]. Based on her body surface area (1.52 m2), combined chemotherapy with TS-1 capsules (50 mg twice daily after breakfast and supper for 21 days) and intravenous cisplatin (60 mg on day 8), followed by 2 drug-free weeks, was administered from the 10th post-operative day. After the termination of 6 cycles of chemotherapy, there was no evidence of tumor recurrence or drug-induced adverse effects. Therefore, single-agent additional maintenance therapy with TS-1 (50 mg twice daily after breakfast and supper for 4 weeks), with 2 weeks of a drug-free interval was continued since 12 December 2008. During the period of administration of TS-1, physical examination and laboratory test were checked every month. Abnormal finding was not detected during this period. Eight months later, she complained of a productive cough with sputum and mild dyspnea on 7 August 2009. The laboratory findings were within normal limits, including a negative sputum culture (gram stain and acid-fast bacillus culture). Fine crackles were audible on auscultation and a chest X-ray showed an interstitial shadow in the lower lung fields bilaterally (Fig. 1). The chest CT scan revealed diffuse ILD, such as idiopathic pulmonary fibrosis or bronchiolitis obliterans with organizing pneumonia in the lung fields bilaterally (Fig. 1). Cessation of chemotherapy and the administration of corticosteroids relieved the patient's symptoms mildly; however, the interstitial shadows persisted on chest X-rays without any improvement. Then, corticosteroid therapy was continued with daily 7.5 mg of prednisolone. On 15 December 2009, a chest X-ray and CT scan was obtained because of the patient's worsening pulmonary symptoms and revealed progression of diffuse ILD (Fig. 2). In spite of the aggressive management in intensive care unit and the intravenous administration of corticosteroids with 125 mg of methylprednisolone, her symptoms did not improve. The pathologic organism was not verified by sputum microbiologic study. She died of pulmonary failure due to progression of interstitial pneumonia on 11 January 2010. An autopsy was not performed.
Instead of the conventional high-dose intravenous administration that has as a goal of total cell killing, the concept of how to continue drug administration safely for long periods and ultimately prolong survival without compromising quality of life is now the standard chemotherapeutic approach for gastric cancer. Thus, TS-1 could be an attractive anti-cancer agent for gastric cancer, with a high response rate and low toxicity. The possibility of outpatient use of TS-1 has increased the convenience for both physicians and patients [3,4].
Several randomized trials have shown that TS-1 is an excellent oral anti-cancer drug for the treatment of advanced gastric cancer, and have suggested that this agent may be useful for neoadjuvant chemotherapy [6].
For outpatient chemotherapy, however, a low incidence of severe adverse reactions is especially important. The incidence of grade III or IV adverse reactions of TS-1 in 449 patients was <10% in phase II clinical trials, except for neutropenia (11.1%). The incidence of grade III or IV non-hematologic adverse reactions, stomatitis, nausea, vomiting, diarrhea and anorexia was low because of the Oxo contained in TS-1. Oxo is distributed in the gastrointestinal tract at a high concentration after oral administration, and alleviates gastrointestinal toxicity. Handfoot syndrome, although not life-threatening, can severely disrupt the patient's daily life; however, hand-foot syndrome was not observed because CDHP reduced the degradation of 5 Flurouracil (5-FU) in the liver [3].
In the early post-operative period, patients have not recovered from surgical stress and the limitation of food intake due to gastrectomy itself could possibly exacerbate the adverse reactions, such as anorexia and nausea. Thus, a delay in the start of drug administration seems necessary to prevent these problems.
Some regimens of combination chemotherapy with high response rates have been recently reported for advanced gastric cancer. A 5-FU and low-dose cisplatin (CDDP) regimen is currently used widely due to its high efficacy [2], and our patient also received it initially.
Drug-induced ILD is not uncommon, ranging from benign infiltration to life-threatening acute respiratory distress syndrome. These clinical patterns differ depending on the patient's illness and drug factors, including the type of drug [7]. A comprehensive knowledge of the mechanisms involved in the development of drug-induced ILD has not been achieved. The patient with suspected ILD presents a diagnostic challenge to physicians. Clinical, radiologic, and pathologic observations are necessary to exclude other conditions with similar symptoms, such as lymphangitis carcinomatosis, pneumonia, allergy, cardiogenic edema, and pulmonary hemorrhage. Thus, a detailed recent drug history is helpful to make the diagnosis.
There has been only one report of ILD induced by TS-1 in patients with gastric cancer [8]. ILD was aggravated after the start of treatment with TS-1 within 1 month. The chemotherapy was discontinued promptly and the patient was treated with corticosteroids for 9 days. The subjective symptoms improved considerably and the interstitial shadows observed on the radiologic evaluations were resolved. Thus, the patient who had an acute onset of pulmonary complications due to TS-1 appeared to have recovered.
The patient in the present report had a different course. She was treated with TS-1 and low-dose cisplatin for 6 months, then continued the single-agent use of TS-1 for 8 months. Cisplatin might not be associated with ILD because there was no evidence of drug-induced adverse effects after the termination of combination therapy.
S-1 combines three pharmacological agents: tegafur, CDHP, and potassium Oxo. These agents maintains a high concentration of 5-FU in the blood and tumors over period of time, although plasma level of TS-1 was not checked. Urinary excretion within 12 hours after administration of TS-1 was 47.4% for CDHP, and 7.0% for 5-FU. Urinary excretion within 72 hours was 52.8% for CDHP, and 7.4% for 5-FU. Urinary excretion was nearly completed by 12 hours and large amounts of 5-FU excrete as CO2 by lung [9]. Therefore, ILD is thought to be caused by a misdirected immune or healing reaction to TS-1 for long time. We assumed that long-term retention and reaction of the drug in the lung would alter the lung parenchyma irreversibly, and might impair resolution on discontinuation of the drug with multiple therapies, including the administration of corticosteroids. Thus, the only method to avoid the progression to life-threatening ILD is prevention or early detection of disease.
One study showed that interstitial shadows on radiologic findings are associated with a risk of ILD in patients with such findings [10]. Therefore, we recommended that a pulmonary radiologic evaluation should be performed before the start of TS-1 for the prevention of ILD. Moreover, routine pulmonary evaluation is inevitable in patients who are prescribed TS-1 to detect the progression of ILD at an early stage, thus providing an opportunity to recover and reduce the drug-associated morbidity and mortality.
In conclusion, TS-1 is a proven anti-cancer agent for gastric cancer with a high response rate and low toxicity. The outpatient use of TS-1 is thus attractive for both physicians and patients. Occasionally, TS-1 can cause irreversible ILD with a long-term retention in the lung that the outcome can be life-threatening. Thus, we have to keep in mind the potential for drug toxicity in the form of ILD. Pulmonary evaluation by meticulous history taking and physical examination and chest X-ray is advisable in patients who are prescribed TS-1 so that it may contribute to a reduction in its morbidity and mortality. We should identity the mechanism underlying the fatal complication of TS-1 and clarify which component of the drug is mostly related to it.
Figures and Tables
References
1. Kim YH. Chemotherapy for advanced gastric cancer: slow but further progress. Cancer Res Treat. 2005. 37:79–86.
2. Tsujitani S, Fukuda K, Kaibara N. Combination chemotherapy of S-1 and low-dose cisplatin for advanced gastric cancer. Gastric Cancer. 2003. 6:Suppl 1. 50–57.
3. Shirasaka T. Development history and concept of an oral anticancer agent S-1 (TS-1): its clinical usefulness and future vistas. Jpn J Clin Oncol. 2009. 39:2–15.
4. Maehara Y. S-1 in gastric cancer: a comprehensive review. Gastric Cancer. 2003. 6:Suppl 1. 2–8.
5. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982. 5:649–655.
6. Mori S, Kishimoto H, Tauchi K, Higuchi K. Histological complete response in advanced gastric cancer after 2 weeks of S-1 administration as neoadjuvant chemotherapy. Gastric Cancer. 2006. 9:136–139.
7. Camus P, Kudoh S, Ebina M. Interstitial lung disease associated with drug therapy. Br J Cancer. 2004. 91:Suppl 2. S18–S23.
8. Kurakawa E, Kasuga I, Ishizuka S, Yoshida T, Kunisawa A, Minemura K, et al. Interstitial pneumonia possibly due to a novel anticancer drug, TS-1: first case report. Jpn J Clin Oncol. 2001. 31:284–286.
9. Hirata K, Horikoshi N, Aiba K, Okazaki M, Denno R, Sasaki K, et al. Pharmacokinetic study of S-1, a novel oral fluorouracil antitumor drug. Clin Cancer Res. 1999. 5:2000–2005.
10. Niho S, Goto K, Yoh K, Kim YH, Ohmatsu H, Kubota K, et al. Interstitial shadow on chest CT is associated with the onset of interstitial lung disease caused by chemotherapeutic drugs. Jpn J Clin Oncol. 2006. 36:269–273.




 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download