Abstract
Purpose
Portal vein thrombosis (PVT) has been considered a relative contraindication for living donor liver transplantation (LDLT). However, it is no longer a contraindication of LDLT due to improvement in surgical techniques and approaches to PVT. The aim of this study was to assess the impact of PVT on outcomes in LDLT patients.
Methods
We retrospectively analyzed the data from 97 adult patients undergoing LDLT in our center from July 2008 to June 2010. Intraoperative findings and preoperative imaging results were reviewed for PVT grading (Yerdel grading). We analyzed the technical aspects and comparisons of risk factors, perioperative variables, and survivals between patients with and without PVT based on the grades.
Results
In the 97 LDLT patients, 18 patients were confirmed to have PVT (18.5%) including grade I cases (n = 8), grade II (n = 7), and grade III (n = 3). Prior treatment of portal hypertension was found to be an independent risk factor for PVT (P = 0.001). The comparisons between PVT and no PVT groups showed no significant difference in intraoperative and postoperative variables except for postoperative bleeding (P = 0.036). The short-term portal vein patency, in-hospital mortality and survival rates were not significantly different between the PVT and control groups.
Conclusion
The outcomes are similar to non-PVT group in terms of in-hospital mortality, survival rates, and postoperative complications. Therefore, our study suggests that PVT cannot be considered to be a contraindication for LDLT and LDLT could be undertaken without increased morbidity and mortality in patients with PVT, in spite of operative complexity.
In the early period of liver transplantation (LT), portal vein thrombosis (PVT) was considered a contraindication for operation because of the technical difficulties it entailed, especially the inability to gain an adequate portal supply [1-3]. In 1985, Shaw et al. [4] reported the first successful deceased donor liver transplantation (DDLT) for a PVT patient and since then, many innovative surgical techniques have been introduced such as thrombectomy, portal vein (PV) reconstruction using vein grafts, and cavoportal hemitransposition [5-9]. Current PVT patients are no longer regarded to be contraindicated for LT and the results for patients with PVT is now comparable to that of patients without PVT [1,2,9,10]. Nevertheless, PVT is considered to add high risk to LT because of the complexity of LT procedures, resulting in significant surgical morbidity and mortality [10,11].
In living donor liver transplantation (LDLT), the issue of subjecting a healthy donor to potentially significant morbidity and mortality has led to a critical reassessment of the recipient selection criteria that are considered acceptable in DDLT [12]. In addition, there are some technical difficulties due to these innovations for preexisting PVT patients; necessity of distal dissection of vascular pedicle of the hilum and restricted availability of deceased donor vein graft [10,11].
Therefore, based on greater technical difficulties and the results from DDLT in this group of patients, the presence of PVT has often been considered to be a relative or absolute contraindication in LDLT [13,14].
From this point of view, the purpose of this report is to analyze single-center experiences in management of PVT, and to assess the impact of PVT on the outcomes in adult LDLT patients.
We retrospectively studied the records of 97 LDLT patients using data collected prospectively among 109 cases of consecutive adult LDLT performed at our center from July 2008 to June 2010. In our center, PVT has not been a contraindication for LDLT except where the preoperative radiologic studies demonstrate a gross tumor thrombus in the main portal vein. All PVT patients were diagnosed using abdominal computed tomography (CT) performed within 3 months before transplantation, and intraoperative detection. For standardization purposes, 12 patients were excluded for the following reasons: 1) no availability of preoperative and postoperative CT scans, 2) insufficient records of the intraoperative findings, or 3) tumor thrombus confirmed by postoperative pathology.
PV flow was monitored routinely after transplantation by Doppler ultrasound at post-transplant days 1,4, and 7, and dynamic CT scan at days 14 and 21.
PVT was diagnosed preoperatively and/or intraoperatively in 18 cases (18.5%). These patients with preoperatively and/or intraoperatively confirmed PVT formed the study group. Patients without PVT and transplanted during the same period were used as the control group.
All patients with confirmed PVT were retrospectively classified into four grades according to the extent of thromboses, as described by Yerdel et al. [15]: Grade I: minimally or partially thrombosed PV, in which the thrombus is mild or, at the most, confined to <50% of the vessel lumen with or without minimal extension into the superior mesenteric vein (SMV). Grade II showed >50% occlusion of the PV, including total occlusion with or without minimal extension into the SMV. Grade III were complete thromboses of both PV and proximal SMV with an open distal SMV. Grade IV was complete thrombosis of the portal vein as well as the proximal and distal SMV.
The preoperative, intraoperative, and postoperative parameters in these two groups were compared. The patients with PVT and those with non-PVT were followed up for a median time of 15.3 months (range, 1.2 to 36.5 months) and 14.9 months (range, 2.4 to 36.3 months).
Analyzed potential risk factors for PVT included; age, sex, primary disease or Child-Turcott-Pugh (CTP) score, the average model for end-stage liver disease (MELD) sore, preoperative platelet count, preoperative prothrombin time, living donor characteristics, quality of donated liver, previous treatment for portal hypertension (splenectomy, shunt operation, transjugular intrahepatic portosystemic shunt [TIPS], or sclerotherapy), and presence of malignancy.
Surgery time, amount of operative red blood cell (RBC) transfusion, duration of hospital and intensive care unit (ICU) stays were analyzed. Postoperative complications (postoperative bleeding, biliary complication, PVT or stenosis, hepatic artery complication, infection, rejection) were compared. In-hospital mortality and patient survival were compared according to the presence of PVT.
Continuous variables are represented as a mean ± SD and categorical data were analyzed using the Fisher's exact test. A Mann-Whitney U test was used to compare the group means. The Kaplan-Meier method was used to estimate survival curves, and survival curves were compared by means of the log-rank test. All analyses were carried out using SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA). A P-value less than 0.05 was considered significant.
PVT was diagnosed in 18 of the total 97 adult patients (18.5%) who underwent LDLT including 8 cases (44.4%) of grade I; 7 (38.9%) of grade II; 3 (16.7%) of grade III.
Age, gender, primary disease, CTP score, MELD score, presence of hepatocellular carcinoma (HCC), ascites, preoperative platelet count, and prothrombin time showed no relationship to PVT (P > 0.05). Living donor characteristics and quality of donated liver had no significant differences between the two groups. However, previous treatment of portal hypertension (splenectomy, shunt operation, TIPS, sclerotherapy) was associated with PVT (P = 0.014).
The mean duration of operation in the patients with PVT (598.2 ± 115.4 minutes) was longer than in those without PVT (572.6 ± 97.6 minutes), but this was not statistically significant (P = 0.483). The overall mean RBC transfusion requirement in the patients with PVT (1,018.2 ± 816.2 mL) was not significantly different to that in those without PVT (1,203.7 ± 1,021.7 mL, P = 0.485). The mean ICU stay was similar in both groups (4.47 ± 1.12 days vs. 5.32 ± 5.6 days, P = 0.632). The mean hospital stay was also similar (32.2 ± 11.6 days vs. 34.5 ± 23.1 days, P = 0.612).
The incidence of postoperative bleeding in the patients with PVT was significant higher than in those without PVT (22.2% vs. 6.3%, P = 0.049). But, the rate of portal vein rethrombosis or stenosis, hepatic artery complication, infection, biliary complication (bile leak, biliary stricture), and rejection were similar in the PVT and non-PVT groups.
The PV patency of the PVT group in their follow-up period was similar to the non-PVT group. Only one of the patients with PVT developed a rethrombosis. A relaparotomy and intraoperative PV stenting were performed at the 13th day after transplantation.
The in-hospital mortality for patients with PVT was similar to that of patients without PVT (5.6% vs. 3.8%, P = 0.548). The 1-and 3-year actuarial survival rate in the PVT group were 87.7% and 75.2%, respectively, but there was no statistical difference between the PVT group and the non-PVT groups (log-rank test, P = 0.357) (Fig. 1A).
Considering the influence of HCC on survival, we excluded patients with cancer from both groups, leaving 36 patients in the PVT group and the controls, still there was no significant difference between the two groups (log-rank test, P = 0.979) (Fig. 1B).
Surgical management of PVT was dependent on the characteristics of the thrombus; its size (partial or complete) or extension degree through the portal venous system. Surgical procedures for PVT are shown in Table 3.
Most patients with grade I and II thrombosis were managed with classic PV-PV anastomosis with or without simple thrombectomy or eversion thrombectomy. In only one patient with grade II, an interposition iliac vein graft was used as anastomosis between the donor's PV to the recipient's proximal portal vein nearby the spleno-portal junction because the hilum had severe fibrotic change due to a previous hepatectomy where the thromboses were not completely removed.
One patient with grade III thrombosis was successfully treated with eversion thrombectomy after lower dissection and classic PV-PV anastomosis. However, the other two cases failed complete eversion thrombectomy, and had no suitable PV to perform the classic anastomosis. In one of these cases, interposition iliac vein graft was used for PV reconstruction. And, in addition, intraoperative PV stenting was performed because there was residual thrombus at the spleno-mesenteric junction and proximal SMV (Fig. 2).
In the other case with complete and extended occlusion of the SMA and a large splenorenal shunt, renoportal anastomosis was performed in a side-to-end fashion (Fig. 3).
The PVT is a well-established complication among patients with end-stage liver disease, and its incidence ranges from 2 to 26% in various centers [8,14], reaching as high as 39% in certain patient populations [16]. The incidence of PVT in LDLT patients at our center was 18.5%.
The etiology is not completely understood, but is believed to be related to anatomic change in the liver owing to the cirrhotic process, increased portal pressure, endothelial injury or coagulation changes [1,15,17,18]. In past studies, high-risk factors for developing PVT included autoimmune hepatitis, cryptogenic cirrhosis, chronic active hepatitis, Budd-Chiari syndrome, male gender, increased age, trauma, hypercoagulative states, Child-Pugh C, and treatment of portal hypertension (splenectomy, shunt operation, TIPS) [4,14,15,19]. But, this study found that previous treatment of portal hypertension was an independent risk factor for PVT prior to LDLT.
PVT was considered for 2 decades to be an absolute contraindication for LT [17]. However, in 1985, two successful liver transplantations were reported despite PVT [4]. Since then, progress in LT has allowed surgeons to utilize multiple techniques including thrombectomy of native veins, extensive thromboendovenectomy up to splenomesenteric confluence, venous interposition graft, renoportal anastomosis, cavoportal hemitransposition for overcoming this major problem and restoring PV flow [6,15,20,21]. But, there are some problems with technical difficulties and restricted availability of vein graft in LDLT as of yet [10,11,18]. The ideal surgical technique to resolve PVT during LT is not defined. The treatment depends on the characteristics of thrombosis (whether acute or chronic), degree (partial or complete), and especially, degree of extension to the splanchnic venous system [1,17].
In our study, 93.3% (n = 14) of patients with grade I and II PVT were successfully managed by classic PV-PV anastomosis with or without simple or eversion thrombectomy. In only one patient with grade II PVT, we performed thrombosed PV resection and portal vein reconstruction using interposition vein graft between the donor's PV and the recipient's proximal PV close to spleno-portal junction because of severe porta hepatis fibrotic change and incomplete eversion thrombectomy.
In one grade III PVT patient eversion thrombectomy failed, interposition vein graft was used and intraoperative PV stenting was performed due to residual thrombus at portomesenteric confluence level. And in the other case of grade III PVT with large splenorenal shunt, we experienced portal vein reconstruction using an interposition iliac vein graft connected to the left renal vein in a side-to-end fashion.
Regarding to blood transfusion, operation time, and in-hospital stay including ICU stay, no statistical difference was observed between the PVT and non-PVT groups in our study, indicating that proper management of PVT is a controllable procedure of LDLT.
The greater technical difficulty in patients with pre-existing PVT has demonstrated an increased risk of complications like hepatic artery thrombosis, relaparotomy, postoperative pancreatitis, sepsis, and renal failure in the different studies [9,15,22]. In our center, comparisons of the PVT patients and controls showed no statistical differences except postoperative bleeding. This increased risk is related to pre-existing PV pathology, high blood loss, the development of coagulopathy and severe acidosis [23]. Therefore, meticulous operative procedures and close monitoring for postoperative bleeding are required.
Initially, patients with PVT undergoing LT showed a high mortality rate in some papers [24]. But, recent studies such as that of Lladó et al. [25] have described similar survival in patients with PVT compared to patients without PVT. In our study, the results (in-hospital mortality, 1-and 3-year survival rates) obtained in patients undergoing LDLT with preoperative PVT are not significantly different to patients without PVT, despite short-term follow-up. Moreover, PVT of grade II to III can be managed with different techniques, with good postoperative results. The results suggest that accurate preoperative evaluation and detailed surgical planning are essential for restoring PV patency in LDLT patients.
In conclusion, the results are similar to non-PVT group in terms of in-hospital mortality, survival rates, and postoperative complications, and PV patency. Therefore, our experience suggests that PVT cannot be considered to be a contraindication for LDLT in spite of operative complexity.
In PVT of grade II and III, LDLT could be undertaken successfully with accurate preoperative diagnosis and proper surgical techniques and good postoperative outcomes can be obtained. Furthermore, innovations of therapeutic approaches and accumulation of experience could be required to improve the outcomes in LDLT with the more extensive PVT patients.
Figures and Tables
Fig. 1
Survival curves in the portal vein thrombosis (PVT) and non-PVT groups (A), and in the PVT and non-PVT groups after excluding malignancy (B).

Fig. 2
Portal vein (PV) reconstruction with interposition vein graft in case of failed eversion thrombectomy. Preoperative computed tomography (CT) scan of this case showed that portal vein is completely obstructed (white arrow) and PV thrombosis propagated to portomesenteric junction (A). Interposition vein graft was used for portal vein reconstruction (B) and intraoperative PV stenting (black arrow) was performed due to residual thrombus (C). Patency of interposition graft was demonstrated by postoperative CT scan (D).
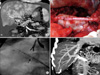
Fig. 3
Portal vein (PV) reconstruction with renoportal anastomosis. Abdominal computed tomography (CT) scan showed complete portal vein thrombosis extended to proximal superior mesenteric vein (arrow) and marked dilated splenorenal shunt drained into the left renal vein (arrow head) (A). Interposition graft was anastomosed to upper border of left renal vein and the graft PV is anastomosed to proximal end of the interposition graft (B). No signs of portal vein system stenosis were visible in postoperative abdominal CT scan (C).
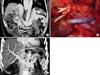
Table 1
Recipient, donor, and graft characteristics of LDLT in patients with and without PVT

Values are presented as mean ± SD or number (%).
LDLT, living donor liver transplantation; PVT, portal vein thrombosis; HBV, hepatitis B virus; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; CTP, Child-Turcott-Pugh; MELD, the average model for end-stage liver disease; INR, international normalized ratio; HTN, hypertension; Y, yes; N, no.
a)Cryptogenic liver cirrhosis, fulminent hepatic failure, primary biliary cirrhosis.
References
1. Tao YF, Teng F, Wang ZX, Guo WY, Shi XM, Wang GH, et al. Liver transplant recipients with portal vein thrombosis: a single center retrospective study. Hepatobiliary Pancreat Dis Int. 2009. 8:34–39.
2. Pan C, Shi Y, Zhang JJ, Deng YL, Zheng H, Zhu ZJ, et al. Single-center experience of 253 portal vein thrombosis patients undergoing liver transplantation in China. Transplant Proc. 2009. 41:3761–3765.
3. Van Thiel DH, Schade RR, Starzl TE, Iwatsuki S, Shaw BW Jr, Gavaler JS, et al. Liver transplantation in adults. Hepatology. 1982. 2:637–640.
4. Shaw BW Jr, Iwatsuki S, Bron K, Starzl TE. Portal vein grafts in hepatic transplantation. Surg Gynecol Obstet. 1985. 161:66–68.
5. Orlando G, De Luca L, Toti L, Zazza S, Angelico M, Casciani CU, et al. Liver transplantation in the presence of portal vein thrombosis: report from a single center. Transplant Proc. 2004. 36:199–202.
6. Molmenti EP, Roodhouse TW, Molmenti H, Jaiswal K, Jung G, Marubashi S, et al. Thrombendvenectomy for organized portal vein thrombosis at the time of liver transplantation. Ann Surg. 2002. 235:292–296.
7. Manzanet G, Sanjuán F, Orbis P, López R, Moya A, Juan M, et al. Liver transplantation in patients with portal vein thrombosis. Liver Transpl. 2001. 7:125–131.
8. Cherqui D, Duvoux C, Rahmouni A, Rotman N, Dhumeaux D, Julien M, et al. Orthotopic liver transplantation in the presence of partial or total portal vein thrombosis: problems in diagnosis and management. World J Surg. 1993. 17:669–674.
9. Dumortier J, Czyglik O, Poncet G, Blanchet MC, Boucaud C, Henry L, et al. Eversion thrombectomy for portal vein thrombosis during liver transplantation. Am J Transplant. 2002. 2:934–938.
10. Cho JY, Suh KS, Shin WY, Lee HW, Yi NJ, Lee KU. Thrombosis confined to the portal vein is not a contraindication for living donor liver transplantation. World J Surg. 2008. 32:1731–1737.
11. Egawa H, Tanaka K, Kasahara M, Takada Y, Oike F, Ogawa K, et al. Single center experience of 39 patients with preoperative portal vein thrombosis among 404 adult living donor liver transplantations. Liver Transpl. 2006. 12:1512–1518.
12. Trotter JF. Selection of donors and recipients for living donor liver transplantation. Liver Transpl. 2000. 6:6 Suppl 2. S52–S58.
13. Kadry Z, Selzner N, Handschin A, Müllhaupt B, Renner EL, Clavien PA. Living donor liver transplantation in patients with portal vein thrombosis: a survey and review of technical issues. Transplantation. 2002. 74:696–701.
14. Davidson BR, Gibson M, Dick R, Burroughs A, Rolles K. Incidence, risk factors, management, and outcome of portal vein abnormalities at orthotopic liver transplantation. Transplantation. 1994. 57:1174–1177.
15. Yerdel MA, Gunson B, Mirza D, Karayalçin K, Olliff S, Buckels J, et al. Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation. 2000. 69:1873–1881.
16. Gayowski TJ, Marino IR, Doyle HR, Echeverri L, Mieles L, Todo S, et al. A high incidence of native portal vein thrombosis in veterans undergoing liver transplantation. J Surg Res. 1996. 60:333–338.
17. Ramos AP, Reigada CP, Ataíde EC, Almeida JR, Cardoso AR, Caruy CA, et al. Portal vein thrombosis and liver transplantation: long term. Transplant Proc. 2010. 42:498–501.
18. Kim SJ, Moon IS, Lee MD, Kim DG. Clinical outcome of preoperative portal vein thrombosis in living donor liver transplantation. J Korean Soc Transplant. 2007. 21:282–290.
19. Nonami T, Yokoyama I, Iwatsuki S, Starzl TE. The incidence of portal vein thrombosis at liver transplantation. Hepatology. 1992. 16:1195–1198.
20. Maluf D, Shim I, Posner M, Cotterell AH, Fisher RA. Salvage procedure for unexpected portal vein thrombosis in living donor liver transplantation. Transplant Proc. 2006. 38:1422–1424.
21. Paskonis M, Jurgaitis J, Mehrabi A, Kashfi A, Fonouni H, Strupas K, et al. Surgical strategies for liver transplantation in the case of portal vein thrombosis: current role of cavoportal hemitransposition and renoportal anastomosis. Clin Transplant. 2006. 20:551–562.
22. Bertelli R, Nardo B, Montalti R, Beltempo P, Puviani L, Cavallari A. Liver transplantation in recipients with portal vein thrombosis: experience of a single transplant center. Transplant Proc. 2005. 37:1119–1121.
23. Shaked A, Busuttil RW. Liver transplantation in patients with portal vein thrombosis and central portacaval shunts. Ann Surg. 1991. 214:696–702.
24. Seu P, Shackleton CR, Shaked A, Imagawa DK, Olthoff KM, Rudich SR, et al. Improved results of liver transplantation in patients with portal vein thrombosis. Arch Surg. 1996. 131:840–844.
25. Lladó L, Fabregat J, Castellote J, Ramos E, Torras J, Jorba R, et al. Management of portal vein thrombosis in liver transplantation: influence on morbidity and mortality. Clin Transplant. 2007. 21:716–721.




 ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download