Abstract
The prognosis of parathyroid carcinoma varies significantly between numerous studies. Therefore, many attempts have been made to grade the degree of parathyroid carcinoma, and recently, classifying parathyroid carcinomas into either minimally invasive or widely invasive carcinoma- similar to follicular carcinoma of the thyroid- has led to a more reliable prediction of the prognosis. Hungry bone syndrome can occur if parathyroidectomy is performed due to primary hyperparathyroidism regardless of the cause of the disease. Hungry bone syndrome is characterized by postoperative a hypocalcemic state due to remineralization of various minerals, including calcium, of the bone; this syndrome requires a long-term supplementation of calcium. The authors aim to report, along with a review of related literatures, 1 case of a 29-year-old female patient diagnosed with minimally invasive parathyroid carcinoma who fell into hungry bone syndrome after parathyroidectomy.
Primary hyperparathyroidism is the third most common endocrine disease [1]. The pathologic causes of hyperparathyroidism can be divided into single adenoma of the parathyroid (89%), multiple hyperplasia of the parathyroid (6%), and parathyroid carcinoma (0.1 to 4%) [2,3].
The diagnosis of parathyroid carcinoma is made by first referring to serum calcium or intact parathyroid hormone (iPTH) levels, then locating the enlarged parathyroid with ultrasonography, scintigraphy, or computed tomography and confirming the parathyroid tumor with fine needle aspiration biopsy. However, if asymptomatic, most parathyroid tumors are adhered to the thyroid gland, and therefore, making the distinction between parathyroid and thyroid tumor is difficult under ultrasonography, and the differential diagnosis is also difficult with cytologic findings alone without clinical signs. Kini et al. [4] stated that vesicle formation and presence of colloid or macrophages suggests thyroid lesions. However, studies on numerous parathyroid lesions have reported that the amount of colloid, vesicular structures, and presence or absence of macrophages, do not greatly help in distinguishing thyroid tumors from parathyroid tumors [5,6]. For this reason, parathyroid carcinomas are often retrospectively diagnosed through surgery and histologically confirmed. The criteria for diagnosis of parathyroid carcinoma include 1) direct invasion into neighboring organs, such as thyroid gland, neck muscles, nerve, and esophagus, 2) distant metastasis to lung or liver, 3) cervical lymph node metastasis, and 4) histological findings including fibrosis, trabecular growth, necrosis, and nuclear atypia (pleomorphism, large nuclei, macronucleoli, mitosis) [7]. The recurrence of parathyroid carcinoma occurs within 2 to 3 years after removal, but the disease-free survival period is known to vary greatly from case to case [8]. Therefore, various studies have attempted subgrouping to classify high-risk and low-risk categories, histologically to reflect variable prognosis of the carcinoma. Among such studies, the study by Bondeson et al. [9] argues that macronucleoli, necrosis, and mitotic activity in excess of 5 per 50 high-power fields reflect bad prognosis. In addition, a report by Kameyama and Takami [10] argues that the criteria suggested by Bondeson et al. did not reflect the patients' prognosisappropriately and classified parathyroid carcinoma into either minimally invasive or widely invasive carcinoma, similar to the classification of follicular carcinoma. The diagnosis of minimally invasive parathyroid carcinoma resulted in benign prognosis.
Serum calcium levels typically returns to normal within 24 to 48 hours after excision of the parathyroid gland due to primary hyperparathyroidism, but hypocalcemia occurs in 10 to 30% of the cases. This is known to be due to the time required for normal parathyroid glands to recover their sensitivity to calcium after surgery, which takes several days [11]. However, persistent hypocalcemia requires differential diagnosis of hypoparathyroidism due to surgery, concomitant hypomagnesemia, and hungry bone syndrome. In other words, after the excision of the parathyroid gland due to primary hyperparathyrodism of any cause, serum iPTH levels normalize and serum calcium and phosphate rapidly migrate to the bone, leading to hypocalcemia. This syndrome is known as hungry bone syndrome, known to occur in about 12.6% of operations on the parathyroid gland [11].
We experienced 1 case of minimally invasive parathyroid cancer accompanied by postoperative hungry bone syndrome, and attempt to report this case along with a review of related literatures.
A 29-year-old female without any notable past history visited for further evaluation of elevated alkaline phosphatase (ALP) and a mass was found at the lower right thyroid gland during a regular checkup. A relatively firm mass of about 3 × 2 cm was found at the right anterior portion of the neck upon examination. The mass was movable, and there was no palpable lymph node.
A clinical laboratory study performed before the operation resulted in the following: blood urea nitrogen 6.3 mg/dL, Creatinine 0.8 mg/dL, serum calcium 11.1 mg/dL, phosphorous 2.1 mg/dL, ALP 1,072 U/L, and iPTH 1,476 pg/dL.
A round, hypoechogenic mass sized about 2 × 1.7 × 3 cm with well-defined margin separate from the right lower thyroid gland was observed under ultrasonography, and accompanied by signs of increased blood flow (Fig. 1). Some lymph nodes appearing to be positive reactive changes were observed inferior to the mass at level VI of right neck. Fine needle aspiration revealed Hurthle cell change of cell clusters, implying the possibility of Hurthle cell neoplasm of the thyroid gland or a parathyroid neoplasm. The immunohistochemistry resultsshowed iPTH and the clinical pattern confirmed the suspicion of parathyroid tumor, and therefore, a diagnosis of parathyroid tumor was made (Fig. 2). Sincecytologic study alone was unable to determine the benign or malignant nature of the tumor, a need for a frozen section of the specimen upon surgical excision was suggested. Imaging studies showed enhancement upon contrast injection, making the determination of the exact nature of the tumor more difficult (Fig. 3).
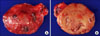
The operation was performed in June 25th, 2010. The right lower parathyroid gland did not show any direct invasion to neighboring organs on surgical examination, and a round shaped mass of about 3 × 2 cm with a smooth capsule easily separable from the thyroid gland and the surrounding muscles was found. The cut surface was yellowish, smooth, and homogenous (Fig. 4). Therefore, a right lower parathyroidectomy was performed, and an excisional biopsy of the surrounding lymph nodes was also performed simultaneously for frozen-sectionevaluation. The pathologic result of the frozen section reported high possibility of benign parathyroidoma, and examination of the excised lymph nodes revealed reactive hyperplasia. Therefore, no further operation was performed. The serum iPTH level immediately after the excision was 199.7 pg/dL, which became 18.54 pg/dL 1 hour later and 43.84 pg/dL 3 hours later.
The patient complained of a tingling sensation 2 days after the operation. Positive Chevostek sign was observed, but the patient did not show any spasmodic muscle contraction. Serum calcium, phosphorous, and iPTH at this period was measured to be 7.2 mg/dL, 1.9 mg/dL, and 102.5 pg/mL, respectively. Intravenous calcium chloride solution and 4 tablets of oral calcium agent (Dicamax 1000 tab, Dalim Biotech, Seoul, Korea) divided into 2 doses twice a day, were concomitantly administered thereafter. The serum calcium level was maintained at below normal value despite a continuous supply of calcium, and serum calcium and iPTH 8 days after operation was 7.1 mg/dL and 226.1 pg/dL, respectively. Dicamax1000 tab was replaced by Healthcal tab (Dong Wha Pharm Co., Seoul, Korea), with a daily dosage of 6 tablets divided into 3 doses three times a day.
The patient was discharged 18 days after operation since she did not show any particular symptoms, and laboratory study performed 174 days after surgery reported iPTH at 65.73 pg/dL, ALP at 172 U/L, serum calcium level at 9.1 mg/dL, and phosphorous at 4.3 mg/dL (Fig. 5). Healthcal tab was replaced by Dicamax 1000 tab with daily dose of 2 tablets divided into 2 doses twice a day.
The final pathologic diagnosis of the patient was minimally invasive parathyroid carcinoma, and no metastasis to surrounding lymph nodes was found (Fig. 6).
Various studies describe cytologic findings that distinguish parathyroid lesions from thyroid lesions, but the distinguishing points vary from paper to paper, and are sometimes even contradictory [4-6,12]. In short, there is no single cytologic finding that can definitively diagnose a parathyroid lesion as of yet. There have been reports of mixing fine needle-aspirated samples of parathyroid with liquid and measuring the concentration of parathyroid hormone of the solution being helpful in making the diagnosis in such cases [5,13]. In the case of this patient, the clinical signs clearly pointed toward parathyroid tumor, and the measurement of parathyroid hormone levels in fine-needle aspirated samples is thought to help in making the diagnosis in cases of asymptomatic parathyroid tumors.
The common causes of hypocalcemia following parathyroidectomy include surgical hypoparathyroidismor hungry bone syndrome. It is especially of major importance to distinguish hungry bone syndrome from surgical hypoparathyroidism in order to start early the appropriate treatment given for a long period [14]. Therefore, we could establishthe cause of hypocalcemia in this patient as hungry bone syndrome.
Hungry bone syndrome was first reported in 1948 after studying patients who showed hypocalcemia, muscle spasm, and hypophosphatemia after receiving parathyroidectomy [15]. Extensive remineralization of the bone, reflecting deposition of calcium and phosphate within the bone parenchyme, was discovered upon bone tissue biopsy, and this finding was named 'hungry bone syndrome'. The treatment of hungry bone syndrome usually involves supplementation of inadequate minerals. Intravenous or oral calcium agents are administered in cases of hypocalcemia, and intravenous or oral magnesium agents are supplied in cases of hypomagnesemia as well. This patient similarly showed hypocalcemia and hypomagnesemia, and therefore, she received supplementary minerals, and a normalized serum calcium level was achieved14 days after operation.
Braiser and Nussbaum [11] suggested relatively large tumor size, blood urea nitrogen, ALP, osteitisfibrosacystica, and old age as risk factors that may cause hungry bone syndrome in patients with primary hyperparathyroidism who receiveparathyroidectomy. When taking into consideration the tumor size and preoperative ALP level, it is thought that the possibility of occurrence of hungry bone syndrome after operation in this patient could have been predicted beforehand. In addition, it is thought that radiographic studies, such as osteitisfibrosacystica, that observe the changes in bone related to hungry bone syndrome may have needed to be performed in more detail.
Mizrachi et al. [16] stated that of all patients with primary hyperparathyroidism due to parathyroid adenoma, 25% experience a postoperative rise of iPTH and that patients with preoperative iPTH greater than 225 pg/mL can be predicted to maintain high levels of iPTH after operation. Mittendorf and McHenry [17] recorded that this postoperative increase of PTH is due to remineralization of the bone, and stated that most postoperative increases of iPTH dropped to normal levels after an average of 16 months. Since this patient recorded preoperative iPTH greater than 225 pg/mL, a persistent elevation in postoperative iPTH could be predicted, and a nearly normal level was recorded after about 6 months of follow-up study. The cause of primary hyperparathyroidism in this case was parathyroid carcinoma, which prompted the possibility of metastatic lesions in distant areas and caused much concern; however, both preoperative and postoperative imaging studies revealed no abnormal findings, so close follow-up monitoring is thought to be needed.
In this patient, a focal, very limited capsular invasion and invasion into one small vessel was histologically observed, which prompted the diagnosis of minimally invasive parathyroid carcinoma according to the aforementioned report by Kameyama and Takami [10]. Their report also stated that all of 12 patients who had no recurrence had minimally invasive parathyroid carcinoma. Therefore, a good prognosis can be expected in this patient as well.
Examination of preoperative laboratory results related to postoperative hungry bone syndrome can help in predicting the possibility of hungry bone syndrome. There is a high possibility that a postoperative increase of iPTH may be caused by remineralization of the bone associated with hungry bone syndrome; also, close follow-up monitoring, including blood tests and imaging studies, is thought to be warranted even in minimally invasive parathyroid carcinomas that are reported to show good prognosis. A case of hungry bone syndrome, accompanied by persisting hypocalcemia and elevated iPTH after operation on postoperatively diagnosed minimally invasive parathyroid carcinoma has been reported as such.
Figures and Tables
Fig. 1
Neck ultrasonography of parathyroid tumor. 2.02 × 1.7 × 2.93 cm sized, well defined, round shaped homogenous and hypoechoic extrathyroidal mass.
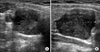
Fig. 2
High power finding of ultrasound guided-fine needle aspiration cytology. (A) The tumor cells forming a microfollicular pattern show slightly enlarged nuclei with mild atypia, abundant cytoplasm and oncocystic change (H&E, ×400). (B) Immunostain result of tumor cell using parathyroid hormone reveals that the origin of tumor cells is parathyroid gland.

Fig. 3
Compute tomographic finding of parathyroid tumor. Right thyroid lobe was displaced anteriorly by well defined heterogenous enhanced mass which was finally reported as a minimally invasive parathyroid carcinoma.
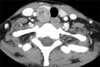
Fig. 4
Gross finding of parathyroid carcinoma case. (A) Tumor were well-encapsulated and external surface were smooth. (B) Tumor was bisected. The cut surface shows white-gray homogenous appearance.
References
1. Melton LJ 3rd. The epidemiology of primary hyperparathyroidism in North America. J Bone Miner Res. 2002. 17:Suppl 2. N12–N17.
2. Wynne AG, van Heerden J, Carney JA, Fitzpatrick LA. Parathyroid carcinoma: clinical and pathologic features in 43 patients. Medicine (Baltimore). 1992. 71:197–205.
3. Chang HS, Yoon JH, Chung WY, Park CS. Coexistence of parathyroid and papillary thyroid carcinoma. J Korean Surg Soc. 2004. 66:147–152.
4. Kini U, Shariff S, Thomas JA. Ultrasonically guided fine needle aspiration of the parathyroid. A report of two cases. Acta Cytol. 1993. 37:747–751.
5. Tseng FY, Hsiao YL, Chang TC. Ultrasound-guided fine needle aspiration cytology of parathyroid lesions. A review of 72 cases. Acta Cytol. 2002. 46:1029–1036.
6. Bondeson L, Bondeson AG, Nissborg A, Thompson NW. Cytopathological variables in parathyroid lesions: a study based on 1,600 cases of hyperparathyroidism. Diagn Cytopathol. 1997. 16:476–482.
7. Shane E, Bilezikian JP. Parathyroid carcinoma: a review of 62 patients. Endocr Rev. 1982. 3:218–226.
8. Shane E. Clinical review 122: parathyroid carcinoma. J Clin Endocrinol Metab. 2001. 86:485–493.
9. Bondeson L, Sandelin K, Grimelius L. Histopathological variables and DNA cytometry in parathyroid carcinoma. Am J Surg Pathol. 1993. 17:820–829.
10. Kameyama K, Takami H. Proposal for the histological classification of parathyroid carcinoma. Endocr Pathol. 2005. 16:49–52.
11. Brasier AR, Nussbaum SR. Hungry bone syndrome: clinical and biochemical predictors of its occurrence after parathyroid surgery. Am J Med. 1988. 84:654–660.
12. Liu F, Gnepp DR, Pisharodi LR. Fine needle aspiration of parathyroid lesions. Acta Cytol. 2004. 48:133–136.
13. Lerud KS, Tabbara SO, DelVecchio DM, Frost AR. Cytomorphology of cystic parathyroid lesions: report of four cases evaluated preoperatively by fine-needle aspiration. Diagn Cytopathol. 1996. 15:306–311.
14. Boeckler P, Grunenberger F, Ruellan A, Vignon F, Weber JC, Bachellier P, et al. Hungry bone syndrome after surgical treatment of severe primary hyperparathyroidism: about 3 cases. Ann Endocrinol (Paris). 2002. 63:8–12.
15. Albright F, Reifenstein EC. The parathyroid glands and metabolic bone disease. 1948. Balltimore: William & Wilkins.
16. Mizrachi A, Gilat H, Bachar G, Feinmesser R, Shpitzer T. Elevated parathyroid hormone levels after parathyroidectomy for primary hyperparathyroidism. Head Neck. 2009. 31:1456–1460.
17. Mittendorf EA, McHenry CR. Persistent parathyroid hormone elevation following curative parathyroidectomy for primary hyperparathyroidism. Arch Otolaryngol Head Neck Surg. 2002. 128:275–279.




 ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download