Abstract
Purpose
The cancer stem cell hypothesis states that the capacity of a cancer to grow and propagate is dependent on a small subset of cells. To determine the significances of the cancer stem cell markers CD133, CD44, and CD24 using a comparative analysis with a focus on tumorigenicity.
Methods
Four pancreatic cancer cell lines, Capan-1, Mia-PACA-2, Panc-1, and SNU-410 were analyzed for the expressions of CD133, CD44, and CD24 by flow cytometry. The tumorigenicity was compared using tumor volumes and numbers of tumors formed/numbers of injection in nonobese diabetic severe combined deficiency mice. Fluorescence-activated cell sorting (FACS) analysis was used to confirm that xenograft explants originated from human pancreatic cancer cells.
Results
CD133 was positive in only Capan-1, CD44 positive in all, CD24 partially positive in
Panc-1. After injecting 2 × 106 cells, all mice administered Capan-1 or Mia-Paca-2 developed tumors, 3 of 5 administered
Panc-1 developed tumors, but no mouse administered SNU-410 developed any tumors. The volumes of Capan-1 tumors
were seven times larger than those of Mia-Paca-2 tumors. When 2 × 105 or 2 × 104 of Capan-1 or Mia-Paca-2 was injected, tumors
developed in all Capan-1 treated mice, but not in Mia-Paca-2 treated mice. Furthermore, xenograft explants of Capan-1
expressed CD133+CD44+ and Capan-1 injected mice developed lung metastasis. FACS analysis showed that xenograft explants
originated from human pancreatic cancer cell lines.
Pancreatic cancer is the ninth most common malignant disease, and is the fifth ranked cause of cancer death in South Korea. Furthermore, advances in surgical and medical therapies have had little impact on the mortality rate of pancreatic cancer, and its 5-year survival rate after curative resection remains dismally low at 10 to 15%. In addition, the mechanisms of local tumor invasion and an early systemic dissemination at the molecular level in pancreatic cancer have not been completely elucidated.
The cancer stem cell (CSC) hypothesis may provide an understanding of cancer development and progression, and a new perspective as to how to develop therapies to treat cancer. CSCs constitute only a small proportion of cancer cells and are capable of self-renewal, differentiation, and tumorigenesis [1]. Research on the existence of CSCs was first undertaken in the context of human acute myeloid leukemia. However, recently studies on the cell surface marker expressions of CSCs, such as, CD133, CD44, CD24, and epithelial-specific antigen (ESA), have reported their involvements in solid malignancies of the breast, brain, prostate, and ovary [2-9].
To date, little study has been conducted on CSCs in pancreatic cancer, and thus, we undertook to determine the significances of cell surface markers, namely, CD133, CD44, and CD24, on the CSCs of pancreatic cancer cells.
The pancreatic cell lines Capan-1, Mia-Paca-2, and Panc-1 were obtained from the American Type Culture Collection (Manassas, VA, USA), and SNU-410 was obtained from the Korean Cell Line Bank (Seoul). All four cell lines were cultured in Roswell Park Memorial Institute medium supplemented with 10% fetal bovine serum (FBS). Explants obtained from nonobese diabetic severe combined immunodeficient (NOD-SCID) mice injected with each of the pancreatic cell lines were cultured in the Dulbecco's modified eagle medium F12 (DMEM/F12) (Hyclone Laboratories Inc., Lakewood, NJ, USA) supplemented with 10% FBS. All pancreatic cell lines originated from the pancreas, and of the four, only Capan-1 was originated from a patient with pancreatic cancer and hepatic metastasis.
For surface marker analysis by flow cytometry, cells were dissociated using trypsin-ethylenediaminetetraacetic acid (EDTA) (trypsin 0.25%; EDTA 0.02%) for 2 to 5 minutes at 37℃, transferred to a 5-mL tube, and washed twice with PBS. Cells were then resuspended in PBS containing 0.1% sodium azide and 2% FBS, anti-human CD133-PE, anti-human CD24-PE, and anti-human CD44-FITC (BD Bioscience, San Jose, CA, USA) were then added, and cells were incubated at 4℃ for 20 minutes and washed twice with PBS. Rat anti-mouse immunoglobulin G (IgG)-fluorescein isothiocyanate (FITC) and Rat anti-mouse IgG-PE were used as isotype controls (BD Biosciences). The origins of explants obtained from xenograft tissues were confirmed by staining with anti-HLA-ABC-FITC (human histocompatibility class I) and anti-H2K-FITC (mouse histocompatibility class I) (BD Bioscience). Dead cells were gated out by propidium iodide (5 µg/mL) staining. Flow cytometry was performed on a FACS, and data were analyzed using Cell Quest software (BD Bioscience).
NOD/SCID mice (aged 6 to 8 weeks) were obtained from Jackson Laboratory (Bar Harbor, ME, USA) and raised under specific pathogen free conditions (Laboratory Animal Research Center, Samsung Medical Center, Seoul).
Single cells from each of the four cell lines were resuspended in 50 µL of PBS, and then injected (2 × 106 to 2 × 104 cells/injection) into the right kidney capsules of NOD/SCID mice. Animals underwent autopsy 6 weeks after implanting 2 × 106 cells. In subsequent studies on Capan-1 and Mia-Paca-2, autopsies were performed 12 weeks after implanting 2 × 104 or 2 × 105 cells (Fig. 1). During autopsies tumor dimensions and volumes, and the presence of any metastatic lesion were noted. Tissues were fixed in formaldehyde and hematoxylin and eosin stain for histologic examinations. In addition, tumor cells from xenografts were confirmed to have originated from the human pancreatic cancer cell lines by FACS. Mice tumors were chopped with a sterile scalpel blade over ice to 2 to 3 mm pieces, transferred to 50 mL tubes, collagenase (1 mg/mL) was then added, and samples were incubated at 37℃ for 1 hour in a shaking incubator. Isolated cells were cultured in Dulbecco's modified Eagle's medium (DMEM)/F12 supplemented with 10% FBS and harvested at passage 1 for FACS analysis.
Cell surface marker expressions were analyzed by FACS. As shown in Fig. 2, 76.5% of Capan-1 cells expressed the cell surface marker CD133, 94% expressed CD44, and 6.9% expressed CD24. For Panc-1, Mia-Paca-2, and SNU-410, few cells were positive for CD133, 94 to 99.9% was positive for CD44, and Panc-1 alone was weakly positive (31.9%) for CD24. Capan-1 was CD133+CD44+CD24-, Mia-Paca-2 CD133-CD144+CD24-, Panc-1 CD133CD44+CD24± and SNU-410 CD133-CD44+CD24-(Table 1).
To compare tumorigenicities, 2 × 106 cells of each pancreatic cell line were injected into NOD/SCID mice (n = 5 per cell line). During autopsy at 6 weeks post-injection, all five mice injected with Capan-1 or Mia-Paca-2 developed tumors, whereas 3 of 5 mice injected with Panc-1 and no mouse injected with SNU-410 developed as tumor. However, Capan-1 tumor volumes were seven times larger than Mia-Paca-2 tumor volumes (Table 2, Fig. 3). In order to identify differences between Capan-1 and Mia-Paca-2, NOD/SCID mice were injected with 2 × 105 or 2 × 104 cells (n = 4 per group) and sacrificed 12 weeks post-injection. In mice injected with 2 × 105 or 2 × 104 Capan-1 cells, all 8 mice developed tumors, where no tumor developed in Mia-Paca-2 injected mice. In addition, metastasis to the lung occurred in Capan-1 treated mice, whereas no metastasis was observed in mice injected with the other cell lines (Table 3, Fig. 4).
Although tumors grown probably originated from the human pancreatic cancer cell lines, it was possible that mice cells could have infiltrated tumors and developed into xenografts. Thus, FACS was used to confirm that xenograft explants obtained originated from the human pancreatic cancer cells. Tumor cells in explants were found to be positive for HLA-ABC but negative for H2K-BDK, indicating that mice cells had not infiltrated tumors. Furthermore, the expressions of cell surface markers in explants were identical to those of the parent cancer cell lines (Fig. 5).
Stem cells reside at the apex of the hierarchical structure that describes the generation of mature cells for particular organs through differentiation. Stem cell biology studies provide an insight into cancer biology, and have led to new approaches in the fight against malignant disease. CSCs are rare, have indefinite self-renewal potential, and drive tumorigenesis. In addition, like their normal stem cell counterparts, CSCs are well suited to survive adverse conditions in the tissue microenvironment [1,10,11]. Recently, it was reported that CSCs are present in acute leukemia [3,6], and in solid malignancies, such as, breast [2], brain [4,5,8], prostate [7] and ovary cancer [9].
Bonnet and Dick [3] first isolated CSCs in myeloid leukemia by using cell surface marker expression and the ability of human leukemia cells to engraft in NOD/SCID mice. The FACS and establishment of human tumor xenograft models in NOD/SCID mice, are important research tools for studies on CSCs [3,12]. Al-Hajj et al. [2] identified CSCs in breast cancer using these tools. They reported that small subset of tumor cells with CD44+CD24-/low epithelial-specific antigen (ESA)+ cell surface marker expression had strong tumorigenicity. Regarding pancreatic cancer cells, heterogeneous surface marker expression for CD44, CD24, and ESA was demonstrated by FACS. These cell surface markers had been shown to be CSC surface markers in previous studies, and are believed to act as adhesive molecules with various signaling functions [13-17]. Li et al. [18] sorted pancreatic cancer cells for CD44, CD 24, and ESA individually and in combination, and reinjected sorted cells into NOD/SCID mice. It was found that cells expressing CD44+CD24+ESA+ comprised only 0.2% to 0.8% of all human pancreatic cancer cells, but had highest tumorigenicity. In the present study, some CD44+CD24+ cell (Mia-Paca-2, Panc-1) also developed tumor after 2 × 106 cells injection, however, when cells of 2 × 105 or 2 × 104 was injected no tumor developed. The cell line study may be the cause of difference compared with the study using pancreatic adenocarcinoma specimen by Li et al [18].
The surface marker CD133 was first reported to have the properties of CSCs in brain and colon cancer [8,19,20]. Hermann et al. [21] demonstrated that CD133+ cells constituted only 1 to 3% of pancreatic adenocarcinoma cells, and found that 500 cells injected in NOD/SCID mice were able to generate tumors. In the literature, two types of CSCs expressing CD44/CD24/ESA or CD133 are present in pancreatic cancer, such findings raise questions regarding the number of types of CSCs and which types have greater tumorigenic potential. Wright et al. investigated this issue in breast cancer and found two distinct types of CSCs expressing CD44+/CD24- or CD133+ and no overlap of surface marker expression. In addition, CSC expressional types have been reported to have similar tumorigenicities [22]. In a study of pancreatic cancer cells, Hermann et al. [21] reported an overlap of 14% between CD44+CD24+ ESA+ and CD133+ pancreatic cancer cells, but unfortunately, no comparison of tumorigenicity was made. Further in vivo tumorigenicity experiments will need to be performed to ascertain if CD44+CD24+ESA+ and CD133+ pancreatic cancer cells represent distinct CSC populations, or if a pancreatic cancer cell population expressing combination of markers is more enriched for CSC function [12,21].
In the present study, we analyzed pancreatic cancer cell lines as for the expressions of CD133, CD44, and CD24, that is, all of the known cell surface markers of CSCs, and these cell lines were also compared with respect to tumorigenicity. Capan-1, which metastasizes to the liver, was only positive for CD133 and CD44 and these two markers may be expressed in the same CSC populations. We performed in vivo experiments on tumorigenicity and found that CD133 positive cells had the highest tumorigenic potential and high rates of metastasis to the lung compared with CD44 positive cells, which suggests that CD133 represents the aggressive behavior of pancreatic cancer. Comparative analysis between CD133 positive cells and CD133 negative cells after sorting from each pancreatic cancer cell lines will support this study and is planned in our labroatory. Hermann et al. [21] suggested that human pancreatic cancer tissue contains CD133 expressing CSCs that are exclusively tumorigenic and associated with metastatic potential.
In the present study, we failed to establish human patient pancreatic cancer xenograft models in NOD/SCID mice, and subsequently switched to pancreatic cancer cell lines. After single cell isolation from human pancreatic cancer tissue, cell was failed to be cultured in vitro. Further research with enough tumor mass is needed to set the microenvironment for in vitro cell culture. However, we realized during the course of this study that animal host cells could infiltrate the tumor. Therefore, we caution that the possibility that xenograft explants have been infiltrated by mice cell should be routinely checked-up by using FACS analysis or karyotyping.
Summarizing, the present study demonstrates: 1) CD133 positive cells had a higher tumorigenic potential than cells expressing CD44, or CD24; 2) Only CD133 positive cells were associated with metastasis to the lung; 3) the tumorigenicity of CD133+ cells was maintained by even a small number of injected cells; and 4) Xenograft explants that developed after injecting CD133+ cells were confirmed to have originated from human pancreatic cancer cells and found to maintain CD133 expression. We suggest that these findings be re-evaluated in freshly retrieved human specimens. Furthermore, our findings suggest that CD133 may be potentially an important cell surface marker of pancreatic cancer stem cells, and that it may be used as a molecular target for the development of treatments for intractable pancreatic cancer.
Figures and Tables
Fig. 1
Overall process of animal study. Animals underwent autopsy 6 weeks after implanting 2 × 106 cells. In subsequent studies on Capan-1 and Mia-Paca-2, autopsies were performed 12 weeks after implanting 2 × 104 or 2 × 105 cells.

Fig. 2
Capan-1 highly expresses CD133 but other cell lines, Panc-1, Mia-Paca-2, SNU-410 express less than 2% of CD133. Panc-1 expresses 30% of CD24 and CD44 double positive but other cell lines express less than 10% of CD24. Most cell lines are CD44 positive.
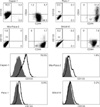
Fig. 3
CD133+ Capan-1 generates a significant tumor growth compared with other CD133-cell lines (A, B). Microscopic finding of xenograft explants of 3 pancreatic cell lines except SNU-410 (C).
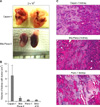
Fig. 4
Tumor formation ability of CD133+ Capan-1 and CD133-Mia-Paca-2 cells were compared after decreasing of number of injected cells. All 133+ Capan-1 cells formed tumors in kidney, whereas no CD133-cell could form tumor. (A, B) Only CD133+ Capan-1 had metastasis to the lung.
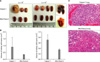
Fig. 5
Characteristics of xenograft explants according to Fluorescence-activated cell sorting analysis. Xenograft explants was also CD133+CD44+ as similar with primary Capan-1. Xenograft explants was positive against anti-HLA-ABC (human histocompatibility class I) but negative against anti-H2K (mouse histocompatibility class I) (bottom).
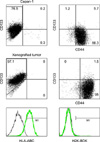
Table 1
Relationships between cell surface marker expression and tumorigenicity in human pancreatic cancer cell lines

References
1. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001. 414:105–111.
2. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003. 100:3983–3988.
3. Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997. 3:730–737.
4. Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004. 64:7011–7021.
5. Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser , et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003. 100:15178–15183.
6. Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994. 367:645–648.
7. Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006. 25:1696–1708.
8. Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004. 432:396–401.
9. Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci U S A. 2006. 103:11154–11159.
10. Bissell MJ, Labarge MA. Context, tissue plasticity, and cancer: are tumor stem cells also regulated by the microenvironment? Cancer Cell. 2005. 7:17–23.
11. McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999. 5:1410–1412.
12. Lee CJ, Dosch J, Simeone DM. Pancreatic cancer stem cells. J Clin Oncol. 2008. 26:2806–2812.
13. Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006. 12:1167–1174.
14. Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006. 442:818–822.
15. Lee SM, Buchler T, Joseph T, Lai C. Bilateral eardrum perforation after long-term treatment with erlotinib. J Clin Oncol. 2008. 26:2582–2584.
16. Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003. 4:33–45.
17. Weichert W, Denkert C, Burkhardt M, Gansukh T, Bellach J, Altevogt P, et al. Cytoplasmic CD24 expression in colorectal cancer independently correlates with shortened patient survival. Clin Cancer Res. 2005. 11:6574–6581.
18. Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007. 67:1030–1037.
19. O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007. 445:106–110.
20. Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007. 445:111–115.
21. Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007. 1:313–323.
22. Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV, Varticovski L. Brca1 breast tumors contain distinct CD44+/CD24= and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008. 10:R10.




 ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download