Abstract
Purpose
Tumors arising from the ampulla of Vater can be benign or malignant. Recently, endoscopic papillectomy has been employed in the management of benign ampulla of Vater tumors; however, surgical resection is the treatment of choice. The aim of this study was to define indications and suggest a role for transduodenal ampullectomy in the management of ampulla of Vater tumors.
Methods
We retrospectively reviewed the medical records of 54 patients treated for ampulla of Vater tumors between January 1999 and December 2008.
Results
Twenty-two endoscopic papillectomies and 21 transduodenal ampullectomies were performed. Four patients underwent transduodenal ampullectomy after endoscopic papillectomy due to a recurrent or remnant tumor. Recurrence or a remnant tumor was found in one patient after transduodenal ampullectomy compared to six patients after endoscopic papillectomy. Immediate intraoperative conversion from transduodenal ampullectomy to pancreaticoduodenectomy was performed in five patients based on intraoperative frozen biopsy analysis.
Conclusion
Transduodenal ampullectomy should be performed to treat ampulla of Vater tumors that are unsuitable for endoscopic papillectomy. Transduodenal ampullectomy can serve as an intermediate treatment option between endoscopic papillectomy and pancreaticoduodenectomy in the management of ampulla of Vater tumors.
Tumors of the ampulla of Vater have various histopathologic characteristics that can be benign or malignant. It is difficult to achieve an accurate diagnosis and exclude malignancy based on preoperative studies because of the possibility of an invasive carcinoma within an adenoma. Endoscopic papillectomy has recently been used to manage periampullary adenomas [1-4], but curative surgical resection remains the treatment of choice [5-7].
Transduodenal ampullectomy was first introduced by Halstead in 1899 [8], and was initially attempted as treatment for ampulla of Vater cancer. The use of this procedure failed to become widespread because the surgical technique had not been standardized and the recurrence rate was high. Since Whipple introduced pancreaticoduodenectomy in 1935 [9], it has been recommended as the definitive surgery for ampulla of Vater lesions [6,7,10]. The performance of transduodenal ampullectomy has been reported in limited cases and in a small number of series. No specific consensus has been reached regarding the indications for transduodenal ampullectomy, and there are few studies that address the role of transduodenal ampullectomy with respect to its relationship with endoscopic papillectomy [11-14]. The aim of this study was to define indications and suggest a role for transduodenal ampullectomy in the management of ampulla of Vater tumors based on a review of experience at a single center .
The medical records of 54 patients with ampulla of Vater tumors treated at the Samsung Medical Center between January 1999 and December 2008 were retrospectively reviewed. All patients underwent an esophagoduodenoscopic (EGD) exam and biopsy. Computed tomography (CT) or endoscopic ultrasonography (EUS) was performed to assess the extent of the tumor. Patients with a malignancy confirmed by preoperative biopsy were excluded from this study.
Patients were initially treated by endoscopic papillectomy, transduodenal ampullectomy, or pancreaticoduodenectomy, depending on tumor size, the presence of high-grade dysplasia on endoscopic biopsy, and suspicions of malignancy on preoperative imaging studies. Endoscopic papillectomy was performed initially in patients with a benign-looking tumor or a tumor size less than 2 cm. Prior to endoscopic papillectomy, a pancreatogram and a cholangiogram were obtained by endoscopic retrograde cholangiopancreatography (ERCP) in order to check for ductal involvement. En bloc resection was attempted in all patients, and when this failed, piecemeal resection was performed. After papillectomy, a pancreatic stent and a biliary stent were inserted to prevent post papillectomy pancreatitis and stenosis. Chest radiography and laboratory tests were performed for all patients to screen for microperforations, pancreatitis, and bleeding after papillectomy. EGD was performed for stent removal 2 to 5 days after initial endoscopic papillectomy. Followup EGD was performed at three to six months intervals for two years, and annual EGD examination was recommended thereafter. If any suspicious mucosal nodularity was found on EGD during follow-up, biopsy was performed and histologic results were reviewed. A remnant lesion was defined as a biopsy-confirmed lesion within a suspicious adenomatous lesion at a previous papillectomy site on subsequent EGD. Recurrence was defined as a lesion detected six months after treatment with at least one EGD biopsy result showing no residual lesion [15].
When patients were not suitable for endoscopic papillectomy (a tumor greater than 2 cm, a tumor with intraductal growth, or a lateral spreading tumor) transduodenal ampullectomy was performed as the initial treatment. Transduodenal ampullectomy was attempted as the initial treatment in patients with any suspicious sign of malignancy on preoperative study, such as high-grade dysplasia on preoperative endoscopic biopsy.
Transduodenal ampullectomy was performed as described in other studies [12,14,16-18]. The operative procedure included the Kocher maneuver, longitudinal duodenotomy, full thickness excision of the ampulla, and re-implantation of the common bile duct and the pancreatic duct into the duodenal wall. Cholecystectomy was performed routinely. Short stents were inserted into the pancreatic ducts to prevent pancreatitis. Tumors, the bile duct and the pancreatic duct margin were sent to pathology for intraoperative frozen section review. Routine lymph node dissection was not performed.
Ten patients who underwent pancreaticoduodenectomy as an initial treatment because of a suspected malignancy on preoperative study, but for whom final pathology revealed a benign tumor, were also included in this study. Patient demographics, symptoms, and preoperative endoscopic biopsy and final pathology findings were reviewed. Post-operative outcomes were evaluated with respect to length of hospital stay, and post-operative complications and recurrence during follow-up.
A total of 54 patients with an ampulla of Vater tumor were included in this retrospective review; 25 were female and 29 were male. Thirty-seven patients were asymptomatic and found to have an abnormality on screening tests such as EGD. The median patient age was 58 years (range, 37 to 75 years). Eighteen patients presented with symptoms including jaundice (8 patients), abdominal pain (7 patients), dyspepsia (2 patients) or general weakness (1 patient) (Table 1). Endoscopic papillectomy was selected as the initial treatment in 22 patients while 32 patients were initially treated with surgery (22 with transduodenal ampullectomy and 10 with pancreaticoduodenectomy) (Fig. 1).
Ten patients had no recurrence after endoscopic papillectomy, and nine patients underwent additional endoscopic papillectomy or ERCP biopsy after endoscopic papillectomy for a remnant adenoma or a positive resection margin (Fig. 1). Two patients had remnant adenoma after the second endoscopic papillectomy and subsequently underwent transduodenal ampullectomy (Fig. 1). Immediate conversion to pancreaticoduodenectomy was performed in one of these two patients because of a positive deep mucosal bile duct margin on intraoperative frozen section analysis (Fig. 1). One patient was found to have a remnant lateral spreading tumor after endoscopic papillectomy. Surgery was recommended, but the patient was unfortunately lost to follow-up (Fig. 1). Two patients eventually underwent transduodenal ampullectomy for a remnant tumor and a pancreatic duct stricture after papillectomy (Fig. 1).
Of the 22 patients for whom transduodenal ampullectomy was initially attempted, 4 patients were converted to pancreaticoduodenectomy after intraoperative frozen biopsy analysis. Of these four patients, three had adenocarcinoma and the other had a carcinoid with a very close margin (Fig. 1).
As a result, 22 patients underwent endoscopic papillectomy while 21 patients underwent transduodenal ampullectomy. Five patients had immediate intraoperative conversion from transduodenal ampullectomy to pancreaticoduodenectomy (Fig. 1). The median hospital stay after endoscopic papillectomy was 5 days (range, 3 to 12 days), which was shorter than the 9 days (range, 7 to 37 days) required after transduodenal ampullectomy (Table 2). The median follow-up period for endoscopic papillectomy was 10 months (range, 1 to 64 months), and that of transduodenal ampullectomy was 18 months (range, 1 to 72 months).
Complications were observed in 11 and 5 patients after endoscopic papillectomy and transduodenal ampullectomy, respectively (Table 3); no deaths occurred after either procedure. The complications that occurred after endoscopic papillectomy were pancreatitis, bleeding, and microperforation. Ten patients with complications had a longer hospital stay than average, but all improved with conservative treatment only. One female patient underwent transduodenal ampullectomy eight months after the initial procedure because of a pancreatic duct stricture. The complications that occurred after transduodenal ampullectomy were delayed gastric emptying, wound dehiscence, passage disturbance, p-duct stenosis-induced pancreatitis, and biliary stricture-induced cholangitis. With the exception of one patient with delayed gastric emptying, the others underwent intervention or surgery for treatment of their complications.
Of the 22 cases that underwent endoscopic papillectomy, 15 had a final pathologic diagnosis of low grade adenoma (Table 4). Pathology results after transduodenal ampullectomy ranged from low grade adenoma to adenocarcinoma (Table 5). The accuracy of preoperative endoscopic biopsy versus final pathology was 47.6% (10 of 21 patients), and the accuracy of intraoperative frozen biopsy analysis versus final pathology was 65.2% (15 of 23 patients); frozen section biopsy analysis was not performed in two patients.
Ampulla of Vater cancer was suspected in one patient with liver cirrhosis due to chronic hepatitis B infection. However, transduodenal ampullectomy was performed instead of pancreaticoduodenectomy because of the risk of postoperative hepatic failure. The ultimate biopsy result in this case was adenocarcinoma with a positive lateral focal deep margin. The patient subsequently underwent adjuvant radiotherapy and there was no evidence of recurrence after 24 months of follow-up.
Five of 22 patients had focal carcinoma or carcinoma in situ according to the final pathology report; all carcinomas were confined to mucosa. One patient with a final pathologic diagnosis of adenocarcinoma within adenomyoma with a negative resection margin experienced recurrence at 33 months postoperatively. A metastatic seeding nodule was found, and this patient was treated with concurrent chemo-radiation therapy.
Screening esophagogastroduodenoscopy has been performed actively over the last decade, and has increased the detection rate of asymptomatic ampulla of Vater tumors [1,2,5,15]. Clinicians are concerned about the proper management of this type of tumor, and radical resection with pancreaticoduodenectomy is widely accepted as the definitive treatment; however the perioperative morbidity and mortality associated with this procedure are problematic [19,20].
Endoscopic papillectomy is an attractive method for treating ampulla of Vater tumors because it reduces the possibility of laparotomy. However, endoscopic papillectomy is inconvenient to patients because frequent endoscopic examinations are required after the procedure [5,21,22]. Furthermore, specimens obtained after piecemeal resection may have inadequate margins or false negative results, therefore close monitoring for recurrent or remnant tumors is recommended. In the present study, three positive resection margins and six remnant tumors were found after 22 endoscopic papillectomies, as compared with one recurrence and one positive resection margin after 21 transduodenal ampullectomies.
The advantage of transduodenal ampullectomy is that it allows complete circumferential resection of the ampulla of Vater, which enables precise pathologic examination. Fig. 2 is a photomicrograph of a specimen obtained after transduodenal ampullectomy with predominantly highgrade dysplasia in the background and a less than 5% focal minimally invasive carcinoma. Had endoscopic papillectomy been performed on this patient, the focal carcinoma would likely have been missed. Accordingly, if it is not possible to obtain a clear resection margin by en bloc resection in endoscopic papillectomy, the patient should undergo transduodenal ampullectomy rather than piecemeal resection. An inadequate resection margin could not only result in tumor recurrence, but may also preclude the chance to diagnosis focal or in situ carcinoma within an adenoma.
The standard procedure for transduodenal ampullectomy includes re-implantation of the bile duct and p-duct, and post-operative pancreatitis is therefore rarely encountered [12,14,16,17,23]. Furthermore, intraoperative frozen section examinations provide another opportunity for immediate conversion to pancreaticoduodenectomy if invasive carcinoma is found. Ten patients underwent pancreaticoduodenectomy initially (Fig. 1) due to suspected malignancy on preoperative study, but final pathologic diagnosis was benign. If transduodenal ampullectomy had been attempted on these patients, pancreaticoduodenectomy could have been avoided.
The limitations of this study are 1) Endoscopic papillectomy has been actively performed at our institute over recent years; 2) Outcomes may have varied depending on the techniques employed by individual endoscopists; and 3) The mean follow-up duration for patients who underwent endoscopic papillectomy or transduodenal ampullectomy was less than 24 months. Further studies with a longer follow-up are required.
In conclusion, transduodenal ampullectomy should be indicated for patients in whom an adequate resection margin was not obtained with endoscopic papillectomy and in patients with high grade dysplasia on endoscopic biopsy. We recommend that transduodenal ampullectomy should be strongly considered for recurrent or remnant tumors after endoscopic papillectomy in order to obtain an adequate resection margin and to provide definite treatment [11-14,24,25]. Because frozen biopsy allows immediate intraoperative conversion to pancreaticoduodenectomy, transduodenal ampullectomy could act as an intermediate step between endoscopic papillectomy and pancreaticoduodenectomy for the treatment of ampulla of Vater tumors (Fig. 3) [26,27]. Use of this technique initially could prevent unnecessary pancreaticoduodenectomy in benign ampulla of Vater tumors [28].
Figures and Tables
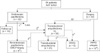 | Fig. 1Diagram of patients flow. ERCP, endoscopic retrograde cholangiopancreatography; PPPD, pancreaticoduodenectomy. a)Remnant tumor, margin (+). b)Remnant tumor. c)Remnant tumor, Lat. spreading type. d)Recurrence, P-duct stricture. e)Frozen: 3 adenocarcinoma, 1 carcinoid very close margin. f)Frozen; deep mucosal bile duct margin (+). g)Final pathology: benign. |
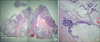 | Fig. 2(A) Microphotograph of specimen of transduodenalampullectomy. Focal minimally invasive carcinoma (<5%) in the background of high grade dysplasia (circle) (H&E, ×10). (B) Magnification view of focal invasive mucinous carcinoma, marked with circle in Fig. 2A (H&E, ×100). |
ACKNOWLEDGEMENTS
This research was supported by grants from IN-SUNG Foundation for Medical Research in 2009 (CA98101).
References
1. Catalano MF, Linder JD, Chak A, Sivak MV Jr, Raijman I, Geenen JE, et al. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc. 2004. 59:225–232.
2. Charton JP, Deinert K, Schumacher B, Neuhaus H. Endoscopic resection for neoplastic diseases of the papilla of Vater. J Hepatobiliary Pancreat Surg. 2004. 11:245–251.
3. Cheng CL, Sherman S, Fogel EL, McHenry L, Watkins JL, Fukushima T, et al. Endoscopic snare papillectomy for tumors of the duodenal papillae. Gastrointest Endosc. 2004. 60:757–764.
4. Yoon SM, Kim MH, Kim MJ, Jang SJ, Lee TY, Kwon S, et al. Focal early stage cancer in ampullary adenoma: surgery or endoscopic papillectomy? Gastrointest Endosc. 2007. 66:701–707.
5. Lee SY, Jang KT, Lee KT, Lee JK, Choi SH, Heo JS, et al. Can endoscopic resection be applied for early stage ampulla of Vater cancer? Gastrointest Endosc. 2006. 63:783–788.
6. Kobayashi A, Konishi M, Nakagohri T, Takahashi S, Kinoshita T. Therapeutic approach to tumors of the ampulla of Vater. Am J Surg. 2006. 192:161–164.
7. Yoon YS, Kim SW, Park SJ, Lee HS, Jang JY, Choi MG, et al. Clinicopathologic analysis of early ampullary cancers with a focus on the feasibility of ampullectomy. Ann Surg. 2005. 242:92–100.
8. Halsted WS. Contributions to the surgery of the bile passages, especially of the common bile duct. Boston Med Surg J. 1899. 141:645–654.
9. Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of Vater. Ann Surg. 1935. 102:763–779.
10. Winter JM, Cameron JL, Olino K, Herman JM, de Jong MC, Hruban RH, et al. Clinicopathologic analysis of ampullary neoplasms in 450 patients: implications for surgical strategy and long-term prognosis. J Gastrointest Surg. 2010. 14:379–387.
11. Fraguela Mariña JA. Transduodenal ampullectomy in the treatment of villous adenomas and adenocarcinomas of the Vater's ampulla. Rev Esp Enferm Dig. 2004. 96:829–834.
12. Dixon E, Vollmer CM Jr, Sahajpal A, Cattral MS, Grant DR, Taylor BR, et al. Transduodenal resection of peri-ampullary lesions. World J Surg. 2005. 29:649–652.
13. Demetriades H, Zacharakis E, Kirou I, Pramateftakis MG, Sapidis N, Kanellos I, et al. Local excision as a treatment for tumors of ampulla of Vater. World J Surg Oncol. 2006. 4:14.
14. Grobmyer SR, Stasik CN, Draganov P, Hemming AW, Dixon LR, Vogel SB, et al. Contemporary results with ampullectomy for 29 "benign" neoplasms of the ampulla. J Am Coll Surg. 2008. 206:466–471.
15. Irani S, Arai A, Ayub K, Biehl T, Brandabur JJ, Dorer R, et al. Papillectomy for ampullary neoplasm: results of a single referral center over a 10-year period. Gastrointest Endosc. 2009. 70:923–932.
16. Meneghetti AT, Safadi B, Stewart L, Way LW. Local resection of ampullary tumors. J Gastrointest Surg. 2005. 9:1300–1306.
17. Maithel SK, Fong Y. Technical aspects of performing transduodenal ampullectomy. J Gastrointest Surg. 2008. 12:1582–1585.
18. Park JS, Yoon DS, Park YN, Lee WJ, Chi HS, Kim BR. Transduodenal local resection for low risk group ampulla of Vater cancer patients. J Korean Surg Soc. 2004. 66:404–408.
19. Böttger TC, Junginger T. Factors influencing morbidity and mortality after pancreaticoduodenectomy: critical analysis of 221 resections. World J Surg. 1999. 23:164–171.
20. Bakkevold KE, Kambestad B. Morbidity and mortality after radical and palliative pancreatic cancer surgery. Risk factors influencing the short-term results. Ann Surg. 1993. 217:356–368.
21. Seewald S, Omar S, Soehendra N. Endoscopic resection of tumors of the ampulla of Vater: how far up and how deep down can we go? Gastrointest Endosc. 2006. 63:789–791.
22. Han J, Kim MH. Endoscopic papillectomy for adenomas of the major duodenal papilla (with video). Gastrointest Endosc. 2006. 63:292–301.
23. Beger HG, Staib L, Schoenberg MH. Ampullectomy for adenoma of the papilla and ampulla of Vater. Langenbecks Arch Surg. 1998. 383:190–193.
24. Branum GD, Pappas TN, Meyers WC. The management of tumors of the ampulla of Vater by local resection. Ann Surg. 1996. 224:621–627.
25. Posner S, Colletti L, Knol J, Mulholland M, Eckhauser F. Safety and long-term efficacy of transduodenal excision for tumors of the ampulla of Vater. Surgery. 2000. 128:694–701.
26. Clary BM, Tyler DS, Dematos P, Gottfried M, Pappas TN. Local ampullary resection with careful intraoperative frozen section evaluation for presumed benign ampullary neoplasms. Surgery. 2000. 127:628–633.
27. Kim JW, Hwang YJ, Kim YI, Yun YK. Transduodenal ampullectomy in ampullary neoplasm. J Korean Surg Soc. 2001. 60:432–437.
28. Tien YW, Yeh CC, Wang SP, Hu RH, Lee PH. Is blind pancreaticoduodenectomy justified for patients with ampullary neoplasms? J Gastrointest Surg. 2009. 13:1666–1673.




 ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download