Abstract
Purpose
This study demonstrated that apoptosis induced by mycophenolic acid (MPA) is mediated by mitochondrial membrane potential transition (MPT) changes in Jurkat cells.
Methods
Cell viability and MPT changes were measured by flow cytometry. Western blotting was performed to evaluate the expression of Bcl-2 family proteins, Bid, truncated Bid (tBid), cytochrome c, voltage dependent anion channel (VDAC), poly ADP-ribose polymerase (PARP), and protein kinase C-δ (PKC-δ). The catalytic activity of caspase-9 and -3 was also measured.
Results
Cell viability was decreased in time- and dose-dependent manners. Bcl-2 protein expression was decreased, but Bax protein expression was identified. A decreased Bcl-XL /Bcl-XS ratio was also noted. The expression of tBid protein also increased in a time-dependent manner in Jurkat cells treated with MPA. While normal MPT appeared as orange fluorescence, abnormal MPT corresponded to green fluorescence. Green fluorescence increased as orange decreased in the MPA-treated cells. Significantly increased concentrations of MPA induced the release of cytosolic cytochrome c. MPA also augmented the catalytic activity of caspase-9 and caspase-3 in Jurkat cells. Our findings demonstrated that MPA-induced apoptosis is mediated by MPT changes accompanied by decreased Bcl-XL expression and the appearance of tBid protein. The release of cytosolic cytochrome c from mitochondria and increased catalytic activity of caspase-9 and caspase-3 were observed in MPA-treated Jurkat cells.
Mycophenolic acid (MPA) is a selective inhibitor of enzymes such as inosine monophosphate dehydrogenase that has a role in guanosine synthesis. MPA also disrupts DNA synthesis in T and B lymphocytes, and suppresses immune functions by inducing apoptosis. However, the underlying signaling pathways are not known. Apoptotic signaling is conducted through the death receptor, mitochondrial, and endoplasmic reticulum-mediated stress protein pathways. Key biochemical events involved in the induction of apoptosis include the up-regulated expression of pro-apoptotic proteins and/or down-regulation of anti-apoptotic proteins. The primary apoptotic signal transduction cascade associated with programmed cell death involves members of the Bcl-2 family [1]. Proteins belonging to the Bcl-2 family either promote cell survival (Bcl-2 and Bcl-XL) or induce apoptosis (Bax) [2]. Increased levels of Bax and/or decreases in Bcl-2 expression leads to the loss of mitochondrial membrane potential. This is a key event in apoptosis induction, and involves a reduction of ATP levels, influx of ions that decreases the mitochondrial membrane potential, and the opening of mitochondrial permeability transition pores [3]. Loss of mitochondrial membrane potential is catastrophic for cells and leads to the release of cytochrome c into the cytosol [3]. Mitochondrial dysfunction has been shown to participate in the induction of apoptosis and has been suggested to activate the apoptotic pathway.
This study evaluated the mitochondrial membrane potential transition (MPT) changes that lead to MPA-mediated apoptosis in Jurkat cells. Cell viability and MPT changes were measured by flow cytometry. Western blotting was performed to evaluate the expression of Bcl-2 family proteins, Bid, tBid, cytochrome c, voltage dependent anion channel (VDAC), and protein kinase C-δ (PKC-δ). The catalytic activity of caspase-9 and -3 in the Jurkat cells was also measured.
Human Jurkat T cells, a T lymphocytic cell line, were obtained from the Korean Collection for Type Cultures (KCTC, Seoul, Korea) and maintained in Roswell Park Memorial Institute medium (RPMI)-1640 (Gibco BRL, Grand Island, NY, USA) tissue culture medium supplemented with 10% fetal calf serum at 37℃ in 5% CO2. MPA (Sigma Chemical Co., St. Louis, MO, USA) was liquefied and dissolved at a concentration of 10 mg/mL in dimethylsufoxide (DMSO), stored at -20℃, and diluted in the RPMI-1640 at final concentration of 0.5 to 100 M/mL.
Cell viability was measured by an 3-[4,5-dimethylthiazol- 2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay. Cells seeded at 100 µL/well in a flat-bottom 24-well plate. The cells were incubated in a CO2 culture medium to permit cell attachment for more than 3 hours and then used for the experiment. An MTT solution (5 mg/mL in phosphate-buffered saline) added at a 1/10 volume of the total cell culture volume. After incubating for 4 hours, 0.01 N HCI with 10% sodium dodecyl sulfate was added (100 µL/well). After the formazan crystals formed by live cells had been dissolved, the absorption of each well was measured by an enzyme-linked immunosorbent assay plate reader (Molecular Devices Co., Sunnyvale, CA, USA) at 540 nm.
Jurkat cells were lysed with lysis buffer (1% Triton X-100, 0.32 M sucrose, 5 mM ethylenediaminetetraacetic acid (EDTA), 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 µg/mL aprotinin, 1 µg/mL leupeptin, 2 mM dithiothreitol (DTT), and 10 mM Tris/HCI, pH 8.0) at 4℃ for 15 minutes, and then centrifuged at 20,000 × g for 15 minutes. The cleared lysates were then treated with bicinchroninic acid (BCA; Sigma Chemical Co.).
The lysates were then incubated with a fluorogenic substrate in a buffer solvent (100 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [HEPES], 10% sucrose, 0.1% Chaps, pH 7.5, 1 mM PMSF, 1 µg/mL aprotinin, 1 µg/mL leupeptin, and 2 mM DTT) at 37℃ for 30 minutes, and fluorescence was measured with a fluorometer (Molecular Devices Co.). Ac-DEVD-AMC (50 µM; Calbiochem, La Jolla, CA, USA) was used as a fluorogenic substrate to measure the enzymatic activity of caspase-3. The level of caspase-3 activity was determined by measuring proteolytic cleavage at a 380 nm excitation wavelength and 460 nm emission wavelength. Ac-LEHD-AFC (50 µM; Calbiochem) was used as a fluorogenic substrate to measure the levels of caspase-9 activity by measuring proteolytic cleavage at a 400 nm excitation wavelength and 505 nm emission wavelength.
After treating Jurkat cells with MPA, the cells were collected and washed twice with cold Hank's balanced salt solution. The cells were lysed in radioimmunoprecipitation assay buffer (50 mM HEPES, pH 7.4; 150 mM NaCl, 1% deoxycholate, 1 mM EDTA, 1 mM PMSF, and 1 µg/mL aprotinin) on ice for 30 minutes. Proteins in the lysates were separated by electrophoresis and transferred to nitrocellulose membranes using a semi-dry electrotransfer system (Illard Co., Seattle, WA, USA) at 4℃ and 30 V for 16 hours. The membranes were incubated in blocking buffer (10% skim milk) at room temperature for 2 hours, and then probed with antibodies against Bcl-2 family proteins, Bid, tBid, cytochrome c, VDAC, PARP, and PKC-δ. The blots were then incubated with a secondary horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at room temperature for 1 hour Immunoreactive bands were detected with an enhanced chemiluminescence kit and by exposing the blots to film.
MPA decreased the viability of Jurkat cells in dose- and time-dependent manners. Bcl-2 protein expression decreased and Bax protein expression appeared following MPA treatment. Additionally, the Bcl-XL/Bcl-XS ratio decreased (Fig. 1). The level of tBid protein also increased in a time-dependent manner in MPA-treated Jurkat cells (Fig. 2). Normal MPT appears as orange fluorescence while green fluorescence indicates abnormal MPT. In the present study, green fluorescence increased while orange decreased in the MPA-treated cells (Fig. 3). Treatment with MPA also significantly increased the release of cytosolic cytochrome c (Fig. 4). Enzymatic activation of caspase-3 and -9 was measured by fluorogenic substrate to examine the mechanism through which apoptosis is induced by MPA. MPA increased the catalytic activity of both caspase- 3 and 9 in Jurkat cells in a time-dependent manner. The enzymatic activation of caspase-9 gradually increased after 6 hours, and reached a maximum (6.5-times greater than the control group) after 30 hours, but rapidly decreased (Fig. 5A). Activation of caspase-3 was also gradually increased (Fig. 5B). The expression of PARP and protein kinase C-δ (PKC-δ) was measured by Western blotting to observe the changes of substrate in the cytoplasm resulting from the activation of caspase-3. A 116 kDa PARP band, one of caspase-3 substrates, was cleaved into a 85 kDa band after 6 hours of MPA treatment; after 24 hours, more than 50% of the PARP had been cleaved. A 78 kDa PKC-δ, another substrate of caspase-3, was cleaved into a 40 kDa fragment after 12 hours of MPA treatment (Fig. 5C). Taken together, our findings demonstrated that MPA increased the catalytic activity of both caspase-9 and -3 in Jurkat cells. We therefore concluded that MPA-induced apoptosis in Jurkat cells is mediated by changes in MPT and the expression of tBid protein via cyotchrome c release and the activity of caspase-9 and -3. Thus, MPA-mediated mitochondrial dysfunction leads to human T lymphocyte apoptosis.
Our study was carried out in human Jurkat cells which share the characteristics of human T lymphocytes and are a well-established model of human T cells. T lymphocytes are a major component of the immune system and are one of the few immune cells found throughout the body. MPA interrupts DNA synthesis in T and B lymphocytes, and suppresses immune function by inducing apoptosis. However, the signaling pathways involved in this procedure are not well understood [4].
Apoptosis, also known as a programmed cell death, is one an essential physiological process involved in the growth of normal organs and constant maintenance of tissues. Hallmarks of apoptosis include cellular contraction of due to dehydration, cell membrane blebbing, increased cytoplasmic calcium concentration, chromatin condensation, DNA fragmentation in a characteristic "ladder pattern" due to activated endonucleases, and apoptotic body formation [5].
The major apoptotic signal transduction cascade involves the Bcl-2 family proteins [6]. Bcl-2 is a protein associated with human follicular lymphoma with a molecular weight of 28 kDa. It is not involved in the cellular growth, but acts to suppress apoptosis. The mechanism by which Bcl-2 prevents apoptosis is known to play a role in controlling the integrity of the outer mitochondrial membrane. A previous study concluded that the decreased ratio of Bcl-XL/Bcl-XS by MPA directly correlated with apoptosis in Jurkat cells [7]. Bcl-2 degradation was observed when cell viability reduced below 20% (40 hours after MPA treatment). In contrast to Bcl-2, Bax is known to promote apoptosis [8]. Expression of Bax was also observed. Thus, our study showed that the expression of Bcl-2 and Bax had opposing effects on apoptosis in Jurkat cells treated with MPA. Increases in the level of Bax and/or decreased Bcl-2 expression leads to loss of mitochondrial membrane potential which is a key event in the induction of apoptosis. This process involves a reduction in ATP levels, influx of ions that leads to decreased mitochondrial membrane potential, and opening of the mitochondrial permeability transition pores [7].
Bid is one of proapoptotic Bcl-2 homology (BH) 3 only proteins. BH3 only proteins promote mitochondrial outer membrane permealization and essential for apoptosis initiation. BH3 only proteins bind with high affinity and specificity to anti-apoptotic Bcl-2 family member, thereby Bax/Bak to elicit mitochondrial outer membrane permealization and activation of caspase cascade [9,10]. tBid targets the mitochondria causing leakage of apoptogenic proteins. Loss of mitochondrial membrane potential is catastrophic for cells and leads to release of cytochrome c into the cytosol [11]. As the level of cytochrome c increases in the cytosol, it interacts with Apaf-1. ATP then forms a complex with procaspase-9, leading to activation of caspase-9 and -3 which results in PARP cleavage [12,13].
Activated caspase-3 cleaves various target protein such as PARP, PKC-δ, lamin, mitogen-activated protein/extracullular signal-regulated kinase, and other caspases. Caspase-3 is central role of apoptosis. It also induces the functional activation or inactivation of the molecular proteins in cellular signaling pathway [14-16]. Our results showed that apoptosis in Jurkat cells treated with MPA was accompanied by activation of caspase-3 and -9 (Fig. 5A, B). The enzymatic activation's increase of caspase-3 and 9 and fragmentation of PARP and PKC-δ (Fig. 5C) were also identified. In summary, we found that MPA-induced apoptosis in Jurkat cells is mediated by MPT changes and the expression of tBid via cyotchrome c release and the catalytic activity of caspase-9 and 3. This result suggests that MPA causes mitochondrial dysfunction that leads to human T lymphocyte apoptosis [17-19].
Figures and Tables
 | Fig. 1Bcl-XL/Bcl-XS ratio decreases in Jurkat cells treated with mycophenolic acid (mycophenolic acid [MPA]; 5 µM). Cells were treated with 5 µM MPA for various periods of time. Equal amounts of protein from cell lysate were separated by 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred onto nitrocellulose membranes, and immunoblotted with anti-Bcl-XL and anti-Bcl-XS antibodies. |
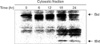 | Fig. 2Mycophenolic acid (MPA) induces the truncated Bid (tBid) protein in Jurkat cells. Cells were treated with 5 µM MPA for various periods and separated into cytosolic and mitochondrial fractions. The cytosolic fraction was separated by 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunoblotted for Bid and tBid. Antibody binding was visualized by enhanced chemilluminescence. |
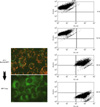 | Fig. 3Mycophenolic acid induces changes in mitochondrial membrane potential transition. Cells were stained with JC-1 and analyzed using flow cytometry. MPT, membrane potential transition. |
 | Fig. 4Mycophenolic acid (MPA) induces the cytosolic release of cytochrome c from mitochondria in Jurkat cells. Cells were treated with 5 µM MPA for various periods of time. Cells were separated into cytosolic and mitochondrial fractions. The two fractions were separated by 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunoblotted for cytochrome c and voltage dependent anion channel (VDAC). Immunoreactive signals were visualized by enhanced chemilluminescence. |
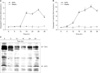 | Fig. 5Mycophenolic acid (MPA) increases the catalytic activity of caspase-9 and -3 of Jurkat cells in a time-dependent manner. Cells were treated with 5 µM MPA for various periods of time and then lysed to measure the activity of these proteases using fluorogenic biosubstrates. The lysate were incubated with Ac-LEHD-AFC to examine caspase-9 (A) or Ac-DEVD-AMC to monitor caspase-3 (B). Protein kinase C-δ (PKC-δ) cleavage was measured by Western blotting with an anti-PKC-δ antibody (C). a)P < 0.05 by student t-test, compared to control group. |
References
1. Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999. 13:1899–1911.
2. Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990. 348:334–336.
3. Crompton M. Bax, Bid and the permeabilization of the mitochondrial outer membrane in apoptosis. Curr Opin Cell Biol. 2000. 12:414–419.
4. Lee WA, Gu L, Miksztal AR, Chu N, Leung K, Nelson PH. Bioavailability improvement of mycophenolic acid through amino ester derivatization. Pharm Res. 1990. 7:161–166.
5. Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter ME. Apoptosis signaling by death receptors. Eur J Biochem. 1998. 254:439–459.
6. Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002. 2:647–656.
7. Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT, Thompson CB. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997. 91:627–637.
8. Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B, et al. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 2005. 24:2096–2103.
9. Huang DC, Strasser A. BH3-Only proteins-essential initiators of apoptotic cell death. Cell. 2000. 103:839–842.
10. Lee YC, Kim JJ, Park HR, Lee S, Woo YM, Cho MH, et al. A study for apoptosis and its mechanism of allogeneic activated T lymphocytes induced by mouse liver immature dendritic cells. J Korean Surg Soc. 2004. 66:1–4.
11. Li PF, Dietz R, von Harsdorf R. p53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c-independent apoptosis blocked by Bcl-2. EMBO J. 1999. 18:6027–6036.
12. Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000. 10:369–377.
13. Gogvadze V, Orrenius S, Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta. 2006. 1757:639–647.
14. Hirata H, Takahashi A, Kobayashi S, Yonehara S, Sawai H, Okazaki T, et al. Caspases are activated in a branched protease cascade and control distinct downstream processes in Fas-induced apoptosis. J Exp Med. 1998. 187:587–600.
15. Creagh EM, Martin SJ. Caspases: cellular demolition experts. Biochem Soc Trans. 2001. 29(Pt 6):696–702.
16. Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999. 15:269–290.
17. Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999. 397:441–446.
18. Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998. 281:1309–1312.
19. Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis. 2003. 8:115–128.




 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download