Abstract
Recently significant neurotoxicity has been reported with the use of carcineurin inhibitors. An 11-year-old-girl had undergone a transplantation of kidney from her mother. On post-operative day 12, hypertension, headache, and left motor weakness (grade I) suddenly occurred. The brain-magnetic resonance imaging and magnetic resonance angiography showed acute cerebral infarction at subcortical white matter of the right hemisphere and multiple stenoses of both anterior cerebral artery and middle cerebral artery. While stopping tacrolimus treatment, we experienced clinical and radiological improvement. So, the neurological complications of this patient seem to have been caused by the use of tacrolimus.
The increasing use of immunosuppressive agents after transplantations has made more complications. Among these, neurological side effects of carcineurin inhibitor are becoming controversial recently. The neurological change related to tacrolimus was first presented as posterior reversible leukoencephalopathy in 1996 [1]. Until now, there have been many clinical symptoms reported and they are referred to various names including posterior reversible encephalopathy syndrome. We report an 11-year-old girl's reversible cerebral infarction and cerebral vascular stenosis after using tacrolimus for her kidney transplantation.
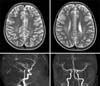
An 11-year-old girl with end-stage renal disease had underwent a transplantation of kidney from her mother. Pre-transplant donor-specific T- and B-cell cross-matches were negative. Initial immunosuppressive treatment consisted of tacrolimus (0.2 ng/kg/day orally, target level 15 to 20 ng/mL), mycophenolate mofetil (1,000 mg twice a day orally), and methylprednisolone (25 mg twice a day orally). Tacrolimus levels remained within the target range during the first ten days. Renal function was quite good after kidney transplantation. On post-operative day 12, hypertension (160/100), headache, and left motor weakness (grade I) suddenly occurred. The brain-magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) findings showed acute cerebral infarction of subcortical white matter of the right hemisphere and multiple stenosis of both anterior cerebral artery (ACA) and middle cerebral artery (MCA) (Fig. 1). Serum tacrolimus level at symptom onset was 19.7 ng/mL. Conservative treatment was done for several days and tacrolimus continued with dosage adjusted to maintain a serum level of 5 to 10 ng/mL. The repeated brain-MRI and MRA scaning was performed at post-operative day 19. The findings showed newly developed acute cerebral infarction on the subcortical white matter of the left hemisphere, cortex of the left parietal lobe and mildly improving status of stenosis in both ACA and MCA (Fig. 2). This neurological change seems to be acute cerebral infarction due to cerebral vascular stenosis caused by either vasculitis or vascular spasm, which is highly suspected to be the consequence of tacrolimus. We decided to change from tacrolimus to cyclosporine. Two days after the change, the cyclosporine level was 232.64 ng/mL. Afterwards, neurologic symptoms improved and follow-up (post-operative day # 29) brain MRI and MRA findings showed an improving status of multifocal acute infarction and no stenosis of either ACA or MCA (Fig. 3). At the 40th postoperative day, left motor weakness improved to grade IV, and rehabilitation treatment was ongoing.
In 1996, Hinchey et al. [1] described a syndrome of acute but reversible clinical findings including headache, mental status alteration, seizures, hypertension, and acute visual changes associated with abnormalities seen on MRI of symmetric white matter lesions, usually in bilateral parietal and occipital lobes. They termed this as reversible posterior leukoencephalopathy syndrome. Although it was initially thought to affect the subcortical white matter only, additional studies supported by improved radiologic imaging modalities have revealed that the cortical gray matter may also be involved. The term "posterior reversible encephalopathy syndrome (PRES)" proposed by Casey et al. [2] is widely used recently because it expresses its clinical manifestation and radiologic findings appro priately. There have been many reports of this syndrome in the literature since its initial definition.
As PRES has become better recognized, many contributing factors have been identified. For example, reports have linked PRES to hypertension, immunosuppressive/chemotherapeutic agents, eclampsia, porphyria, and renaldysfunction [3,4]. The incidence of neurotoxicity, which is one of the major adverse events of calcineurin inhibitors, was higher in patients receiving tacrolimus rather than cyclosporine. As both sensory and motor functions may be adversely affected, patients thus present with a wide range of neurological and psychiatric disorders. Mild symptoms include tremor, neuralgia, and peripheral neuropathy. Severe symptoms could manifest as psychoses, hallucinations, cortical blindness, seizures, cerebellar ataxia, motor weakness, or PRES. MRI is the standard modality in diagnosing this syndrome. Fluid-attenuated inversion recovery is the most sensitive sequence for recognition of cortical and subcortical edema in PRES where hyperintense signal alterations are more prevalent than in conventional sequences [5,6]. This girl showed slightly different radiologic findings from those of typical PRES what causes vasogenic edema with vasculopathy, such as vasoconstriction and vasodilation. This girl's case was unique since there was cytotoxic edema with vasospasm. However, we could experience clinical and radiological improvement when we stopped tacrolimus treatment. Thus, the neurological complication of this patient seems to have been caused by the use of tacrolimus. PRES induced by tacrolimus can usually be diagnosed on the basis of a characteristic clinical and radiographic pattern and is usually reversible by reducing the dosage or with holding the drug for a few days [7]. Failure to recognize its heralding symptoms may potentially increase morbidity in organ transplantation. Therefore, careful examination of patients receiving immunosuppressive agents was needed to discover this uncommon but good prognostic complication.
Figures and Tables
Fig. 1
Brain-magnetic resonance imaging and magnetic resonance angiography examination on post-operative day 12 showed acute cerebral infarction at subcortical white matter of right hemisphere and multiple stenoses of both anterior cerebral artery and middle cerebral artery.
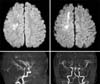
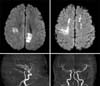
Fig. 2
Brain-magnetic resonance imaging and magnetic resonance angiography examination on post-operative day 19 showed newly developed acute cerebral infarction on subcortical white matter of left hemisphere, cortex of left parietal lobe and mildly improving status of stenosis in both anterior cerebral artery and middle cerebral artery.
References
1. Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996. 334:494–500.
2. Casey SO, Sampaio RC, Michel E, Truwit CL. Posterior reversible encephalopathy syndrome: utility of fluid-attenuated inversion recovery MR imaging in the detection of cortical and subcortical lesions. AJNR Am J Neuroradiol. 2000. 21:1199–1206.
3. Singh N, Bonham A, Fukui M. Immunosuppressive-associated leukoencephalopathy in organ transplant recipients. Transplantation. 2000. 69:467–472.
4. Shin RK, Stern JW, Janss AJ, Hunter JV, Liu GT. Reversible posterior leukoencephalopathy during the treatment of acute lymphoblastic leukemia. Neurology. 2001. 56:388–391.
5. Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2008. 29:447–455.
6. McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, et al. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol. 2007. 189:904–912.
7. Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008. 65:205–210.




 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download