Abstract
Adenomyoepithelioma (AME) is a rare benign tumor composed of myoepithelial cells (MECs) which are located beneath the epithelial cells of exocrine glands, especially in breast and salivary glands. These tumor cells show biphasic proliferation of epithelial and MECs. Malignant AME is characterized by distant metastasis, local recurrence, cytologic atypia, high mitotic activity and infiltrating tumor margins. A 51-year-old woman presented with an 8 months growth in the left breast. She underwent core-needle biopsy and consecutively mammotome assisted biopsy at a local clinic. After resection, she complained about re-growing remnant lesion and a newly developed solid mass in the right breast. Finally, the remnant mass in the left breast was diagnosed with myoepithelial carcinoma. Concurrently, contralateral breast mass was diagnosed with invasive micropapillary carcinoma. Herein we report an unusual case of synchronous myoepithelial carcinoma and invasive micropapillary carcinoma of the breast with a review of literatures.
Adenomyoepithelioma (AME) of the breast is an uncommon tumor characterized by dual epithelial and smooth muscle differentiation of tumor cells [1]. Breast tumors with myoepithelial differentiation are rare but in salivary glands, more common. Sarkar and Lallenbach [2] were first described about various amounts of myoepiepithelial cells with the degree of differentiation of various breast tumors in 1966. Hamperl [3] was first reported to AME of the breast in 1970. According to many reports up to the present, it can demonstrate pure myoepithelial or epi-myoepithelial differentiation of benign and malignant breast tumors [1-3]. Most of the myoepithelioma or AME have been considered to be benign. However, malignant transformation of tumor is characterized by infiltrating tumor margins, atypical cytologic change, brisk of mitotic figures, tumor necrosis and rarely can be distantly metastasized.
According to World Health Organization, myoepithelial lesions of the breast can be classified as myoepitheliosis, adenomyoepithelial adenosis, AME and malignant myoepithelioma (Table 1) [4]. We report herein on a case of synchronous myoepithelial carcinoma arising in AME and invasive micropapillary carcinoma in both breasts.
A 51-year-old woman presented a re-growing remnant mass in the lower central area of left breast, which had already been resected with a mammotome assisted biopsy 6 months ago. Initially, the tumor was recognized as fibroepithelial tumor with fibromatosis. As time went by, the remnant mass started re-growing in the left breast and a newly developed solid mass in the upper outer quadrant of the right breast was found. Her past medical history or family history of breast cancer was unremarkable. Mammography showed a 0.9 cm sized spiculated, heterogenous nodule at lower central portion of the right breast (Fig. 1A). Also a 1.0 cm sized lobulated, iso-density nodule at upper outer quadrant of the right breast was noticed (Fig. 1B). On ultrasound examination, a 1.0 cm sized inhomogeneous, irregular marginated and hypoechoic mass was located at the 5 o'clock direction of the left breast (Fig. 1C) and a 0.9 cm sized multilobulated, heterogenous and hypoechoic mass at 9 o'clock direction of the right breast. Breast conserving surgery with sentinel lymph node biopsy was performed for the left breast mass. At a same time, wide excision was executed for the right breast mass. Clear resection margins and no evidence of tumor metastasis in the sentinel lymph node were found in the submitted specimens. Myoepithelial carcinoma and invasive micropapillary carcinoma were found in the left and right breast, respectively.
Grossly, the tumor mass from the left breast showed a relatively well-demarcated, multinodular, pale tan to white, and solid consistency with partially infiltrating margins.
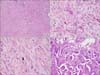
Microscopically, the tumor from the left breast was composed of a central lesion with glandular epithelial lined spaces with increased clear changed myoepithelial cells and surrounding predominantly atypical spindle cells at the left lower corner (Fig. 2A). At the high power field of light microscopy, the atypical tumor cells showed round to spindle, hyperchromatic and pleomorphic nuclei with relatively abundant eosinophilic cytoplasm (Fig. 2B). Atypical mitotic figures were easily found throughout the tumor and up to 8 mitoses were counted in 10 high-power fields (Fig. 2C). The right breast mass consisted of small clusters of tumor cells within the clear stromal spaces (Fig. 2D).
Immunohistochemically, spindle tumor cells were diffusely positive for smooth muscle actin (Fig. 3A), calponin (Fig. 3B), p63 (Fig. 3C) and pancytokeratin (Fig. 3D). The labeling index of Ki-67 at the peripheral portion of mass was more than 40%. On the basis of the histological and immunohistochemical results, we considered this case as a synchronous myoepithelial carcinoma arising in AME and invasive micropapillary carcinoma in both breasts.
AME of the breast is characterized by microscopically biphasic proliferation of epithelial and myoepithelial cells. The AME of breast is a very rare tumor that was already reported in 1970 [3]. The majority of AME are benign, but malignant transformation may occur in AME. Both benign and malignant AME are inclined to local recurrence after surgery and may even recur several years after the initial surgery [5,6]. The guidelines of malignant AME have been not established yet. However we can use the term 'malignant AME' that has infiltrating margins, markedly increased mitotic counts and distinctly cytologic atypia in case of not having definite metastasizing evidence [7]. Also distant metastasis and local recurrence are the most obvious evidences of malignancy. Subsequently, malignant transformation of AME can be divided into three different types according to the main malignant cell types: 1) completely epithelial type; 2) malignant spindle cell type; or 3) both epithelial and myoepithelial type. Malignant epithelial carcinoma arising AME is similar to infiltrating ductal carcinoma, mucoepidermoid carcinoma or adenosquamous carcinoma. Myoepithelial carcinoma or malignant myoepithelioma is purely composed of infiltrating myoepithelial cells with predominantly spindle features.
Microscopically, myoepithelial carcinoma shows infiltrating patterned spindle tumor cells with prominent cytologic atypia, increased mitotic activity (more than 3-4/10 HPF) and definitely infiltrating tumor borders. On immunohistochemistry, spindle tumor cells represent strong positivity of myoepithelial markers, such as smooth muscle actin, calponin, S-100 or p63, and of epithelial markers, such as cytokeratin or epithelial membrane antigen.
Treatment of malignant AME or myoepithelial carcinoma has not been established yet except only complete surgical resection because of the rarity of this tumor. Up to date, several cases of local recurrence or metastasis have been reported so myoepithelial carcinoma should be carefully examined and needed to close follow up. The effectiveness of adjuvant Tamoxifen therapy has not come out.
The differential diagnosis of myoepithelial carcinoma should be included spindle cell carcinoma, fibromatosis and variety of myofibroblastic tumors. The obviously atypical spindle cells which show positivity of myoepithelial and epithelial markers with infiltrating margins are helpful in distinguishing from the spindle cell carcinoma and myofibroblastic tumors. Our case showed also biphasic pattern of immunohistochemistry of strong positivity for myoepithelial markers, such as smooth muscle actin, calponin, p63, and for epithelial marker, such as pancytokeratin. Also it demonstrated definite infiltrating tumor margins with high mitotic rates (4-5/10 HPF) and prominent cytologic atypia. So it could be diagnosed with myoepithelial carcinoma. Also there was a central lesion which showed biphasic proliferation of inner epithelilal and outer myoepithelial cells which could be diagnosed with AME. Consequently, our case is a myoepithelial carcinoma arising in AME. Interestingly, in our case, a contralateral invasive micropapillary carcinoma was found.
Han et al. [8] researched molecular abnormalities of malignant AME and reported the point mutation of p53 gene in this myoepithelial cells, but not in intraluminal epithelial cells or adjacent normal ductal epithelium. Also Jones et al. [9] were performed comparative genomic hybridization (CGH) analysis on a malignant AME case in another study. Furthermore, Angèle et al. [10] have also reported that p53 protein was negative in benign myoepithelial lesions but overexpressed in 44.4% of malignant myoepithelial tumors of the breast. Recently some molecular studies of AME have been trying to identify the molecular pathway of tumorigenesis of AME, but due to the rarity of AME or malignant AME, the pathway has not been established yet.
In conclusion, our case is a rare case of myoepithelial carcinoma arising in AME with contralateral invasive micropapillary carcinoma. Despite the definite diagnosis of malignant AME can be possible throughout various immunohistochemistry, pathologists who are unaware of myoepithelial lesions can make a misdiagnosis of malignant AME. Finally, in case of malignant AME or malignant myoepithelial tumors, close follow up and in some cases, adequate further treatment with adjuvant chemotherapy or radiotherapy should be considered.
Figures and Tables
Fig. 1
(A) Right mammogram showed a spiculated and heterogenous nodule without a focal lesion or microcalcification. (B) Left mammogram demonstrated a lobulated and iso-density nodule. (C) Preoperative ultrasonogram of the left breast revealed a 1.0 cm sized inhomogeneous, irregular marginated and hypoechoic mass.
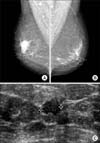
Fig. 2
Microscopic findings of the left breast mass (A-C) and the right breast mass (D). (A) The central lesion of the left breast mass demonstrated adenomyoepithelioma with atypical spindle cells at the left lower corner (H&E, ×100). (B) The atypical spindle tumor cells showed round to spindle, hyperchromatic and pleomorphic nuclei with relatively abundant eosinophilic cytoplasm (H&E, ×400). (C) Atypical mitotic figure (arrow) was found (H&E, ×400). (D) Invasive micropapillary carcinoma was identified in the right breast (H&E, ×200).
References
1. Tavassoli FA. Myoepithelial lesions of the breast. Myoepitheliosis, adenomyoepithelioma, and myoepithelial carcinoma. Am J Surg Pathol. 1991. 15:554–568.
2. Sarkar K, Kallenbach E. Myoepithelial cells in carcinoma of human breast. Am J Pathol. 1966. 49:301–307.
3. Hamperl H. The myothelia (myoepithelial cells). Normal state; regressive changes; hyperplasia; tumors. Curr Top Pathol. 1970. 53:161–220.
4. Tavassoli F, Devilee P. Pathology and genetics of tumours of the breast and female genital organs. 2003. Geneva: World Health Organization.
5. Loose JH, Patchefsky AS, Hollander IJ, Lavin LS, Cooper HS, Katz SM. Adenomyoepithelioma of the breast. A spectrum of biologic behavior. Am J Surg Pathol. 1992. 16:868–876.
6. Ahmed AA, Heller DS. Malignant adenomyoepithelioma of the breast with malignant proliferation of epithelial and myoepithelial elements: a case report and review of the literature. Arch Pathol Lab Med. 2000. 124:632–636.
7. Tavassoli FA. Pathology of the breast. 1999. 2nd ed. Stamford: Appleton & Lange.
8. Han B, Mori I, Nakamura M, Wang X, Ozaki T, Nakamura Y, et al. Myoepithelial carcinoma arising in an adenomyoepithelioma of the breast: case report with immunohistochemical and mutational analysis. Pathol Int. 2006. 56:211–216.
9. Jones C, Tooze R, Lakhani SR. Malignant adenomyoepithelioma of the breast metastasizing to the liver. Virchows Arch. 2003. 442:504–506.
10. Angèle S, Jones C, Reis Filho JS, Fulford LG, Treilleux I, Lakhani SR, et al. Expression of ATM, p53, and the MRE11-Rad50-NBS1 complex in myoepithelial cells from benign and malignant proliferations of the breast. J Clin Pathol. 2004. 57:1179–1184.




 ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download