Abstract
Undifferentiated carcinoma with osteoclast-like giant cells is a rare neoplasm of the exocrine pancreas. Some similar cases have been reported, but the histogenesis of these tumors varies and is controversial. We report here on a case of undifferentiated carcinoma of the pancreas with osteoclast-like giant cells. A 77-year old woman presented with abdominal pain and anorexia. Abdominal computed tomography and magnetic resonance imaging showed an approximately 10 × 5 cm highly attenuated mass arising from the tail of the pancreas and invading the spleen and adjacent bowel loop. The initial impression was a malignant endocrine tumor or solid-pseudopapillary tumor of the pancreas. The patient underwent a distal pancreatectomy with splenectomy and left hemicolectomy. The histopathology and immunohistochemistry helped make the diagnosis that of an undifferentiated carcinoma with osteoclast-like giant cells of the pancreas.
Pancreatic undifferentiated carcinoma with osteoclast-like giant cells is a rare neoplasm that comprises <1% of all exocrine pancreatic tumors [1]. Less than 50 such cases have currently been reported in the literature. This neoplasm is composed of two distinct cell populations: a mononuclear cell population and osteoclastic tumor giant cells of an uncertain lineage [2], and this tumor frequently shows an inhomogenous appearance with cystic structures. The pathogenesis of these tumors is still controversial. Here we report on a case of undifferentiated carcinoma of the pancreas with osteoclast-like cells. The tumor was diagnosed according to the results of histopathological and immunohistochemical studies.
A 77-year-old woman presented with abdominal pain and anorexia for the past month. Her history was otherwise noncontributory. The clinical examination revealed tenderness in the right upper abdominal quadrant and rebound tenderness in the lower abdomen. Blood tests demonstrated elevated transaminase and lipase levels (aspartate aminotransferase 75 IU/L; normal 7 to 38 IU/L, lipase 63 U/L; normal 7 to 60 U/L). The level of carbohydrate antigen 125 was elevated at 279.5 U/mL. The serum concentrations of carcinoembryonic antigen, carbohydrate antigen 19-9 and alpha-fetoprotein were within the normal limits. Abdominal computed tomography (Fig. 1) and magnetic resonance imaging (MRI) (Fig. 2) revealed the presence of a large (about 10 × 5 cm) mass arising from the tail of the pancreas that had invaded the spleen and the adjacent bowel loop. The wall of the tumor was slightly enhanced after administering intravenous contrast medium. No regional lymphadenopathy, ascites, or metastasis was demonstrated on MRI. We performed s distal pancreatectomy with s splenectomy. On the operative field, the pancreatic mass had invaded the adjacent colon and posterior wall of the stomach. Therefore, a left hemicolectomy and partial resection of the stomach were performed together. The patient had an uneventful recovery and she was discharged on postoperative day 11. She refused any additional adjuvant chemotherapy. She was lost to follow-up and died due to pneumonia 3 months after surgery.
The resected specimen was fixed in 20% formalin, and tissue blocks prepared from the tumor were embedded in paraffin. The sections were stained with hematoxylin and eosin for light microscopy. Immunohistochemical studies using avidin-biotinylated peroxidase complex were performed. Antibodies against cytokeratin 19, vimentin, and CD68 were used.
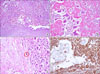
Histologically, the tumor was composed of two major cell types: atypical mononuclear round cells and abundant osteoclast-like multinucleated giant cells with central nucleoli. The atypical cells showed eosinophilic and partially granular cytoplasm, anisokaryotic, hyperchromatic nuclei, and prominent nucleoli (Fig. 4A, B). There were many mitotic figures (Fig. 4C). These atypical cells were generally distributed among the osteoclast-like giant cells without any epithelioid structures. The osteoclast-like giant cells lacked features of atypia and they occasionally showed phagocytosis of the atypical cells.
An immunohistochemical examination showed positive staining for vimentin (Fig. 4D) and CD68 and focal staining by cytokeratin 19 and actin. No staining of C-kit, desmin, CD34 and bcl-2 was observed. The tumor was diagnosed as an undifferentiated carcinoma with osteoclast-like giant cells of the pancreas.
Undifferentiated carcinoma with osteoclast-like giant cells was formerly known as osteoclast-like giant cell tumor; this is a rare neoplasm of the pancreas that is usually diagnosed after s pancreatectomy [1,3].
The nature and origin of osteoclast-like giant cells remain controversial. Epithelial [4], histiocytic [5], and mesenchymal metaplasia [6] have all been suggested. Although the histogenesis of undifferentiated carcinoma with osteoclast-like giant cells has been previously investigated by immunohistochemical, ultrastructural, and molecular biologic studies, the precise mechanisms of the pathophysiology are unknown.
The main signs and symptoms are abdominal pain, a palpable mass, weight loss, fatigue, anorexia and jaundice. Based on our review of the English medical literature [7,8], undifferentiated carcinoma with osteoclast-like giant cells tends to present in the seventh decade of life, although younger patients have been reported. There is no gender predilection. Undifferentiated carcinoma with osteoclast-like giant cells is commonly seen as a large cystic neoplasm with various extents of hemorrhage and necrosis. The head and body portions of the pancreas tend to be involved. In our case, the mass was located in the tail of the pancreas.
The presence of non-neoplastic osteoclast-like giant cells is the histological hallmark of this tumor and the diagnosis is usually not difficult to make when examining tissue sections. Some reports have stated that endoscopic ultrasonography-guided fine needle aspiration biopsy is an effective, accurate means for making the cytological diagnosis of undifferentiated carcinoma with osteoclast-like giant cells of the pancreas [9]. Osteoclastic giant cells rarely express epithelial markers, but they show staining for histomonocytic markers (CD68) [1].
The differential diagnosis of pancreatic undifferentiated carcinoma with osteoclast-like giant cells includes cystic lesions, such as pancreatic cystadenomas, cystadenocarcinomas, serous and mucinous cystic tumors, and pancreatic pseudocysts, and solid pancreatic tumors, such as ductal pancreatic carcinomas or neuroendocrine tumors [8].
Although it is difficult to determine a treatment modality because of the rarity of this tumor, the treatment choice is surgical resection, if possible. In previous reports, adjuvant treatments, including chemotherapy or radiotherapy, were not described, so no detailed information is available on this aspect.
The prognosis of undifferentiated carcinoma with osteoclast-like giant cells is poor, but it is better than that of ductal carcinoma and pleomorphic giant cell tumor of the pancreas, suggesting that undifferentiated carcinoma with osteoclast-like giant cells grows more slowly, and lymph node spread is rare [10]. Recent case reports on this disease suggest that outcomes are improving, although survival is very short for some patients.
In conclusion, undifferentiated carcinoma with osteoclast-like giant cells of the pancreas is an infrequently encountered neoplasm. This tumor can display various clinical characteristics, and its histogenesis is controversial. We suggest that further research based on a large study population is necessary to clarify the histogenesis of this tumor.
Figures and Tables
Fig. 1
Abdominal computed tomography (CT) findings. Abdominal CT scan reveals about a 12 × 10 cm-sized heterogenous enhanced mass arising from the tail of the pancreas. The mass has invaded the spleen and the adjacent bowel loop.
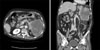
Fig. 2
Abdominal magnetic resonance imaging findings. (A) The T1-weighted image shows a 10 × 5 cm, low signal intensity mass with invasion into the spleen. (B) The T2-weighted image shows heterogenous high signal intensity with multifocal cystic lesions.
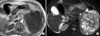
Fig. 3
Gross findings of undifferentiated carcinoma with osteoclast-like giant cells of the pancreas. Gross pathologic examination reveals a 14 × 7.7 cm-sized mass in the pancreatic tail. The cut surface of the tumor is yellowish-white, and the tumor shows signs of hemorrhage and fibrosis.
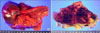
Fig. 4
Microscopic findings. Histologically, the tumor was composed of two major cell types: atypical mononuclear round cells and abundant osteoclast-like multinucleated giant cells with central nucleoli (A, H&E, ×100; B, H&E, ×200). Many mitotic figures are present (C, red circle) (H&E, ×200). The immunohistochemical findings show positive staining by vimentin (D, ×200).
References
1. Molberg KH, Heffess C, Delgado R, Albores-Saavedra J. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas and periampullary region. Cancer. 1998. 82:1279–1287.
2. Bauditz J, Rudolph B, Wermke W. Osteoclast-like giant cell tumors of the pancreas and liver. World J Gastroenterol. 2006. 12:7878–7883.
3. Moon YH, Chu YC, Kim KR. Giant cell carcinoma of the pancreas of the osteoclastic type associated with a mucinous cystadenocarcinoma. J Korean Surg Soc. 1989. 36:546–551.
4. Verbeke CS, Menon KV. Osteoclast-like giant cell tumour of the pancreas: an undifferentiated carcinoma of duct epithelial origin. Pancreatology. 2006. 6:254.
5. Sakai Y, Kupelioglu AA, Yanagisawa A, Yamaguchi K, Hidaka E, Matsuya S, et al. Origin of giant cells in osteoclast-like giant cell tumors of the pancreas. Hum Pathol. 2000. 31:1223–1229.
6. Nai GA, Amico E, Gimenez VR, Guilmar M. Osteoclast-like giant cell tumor of the pancreas associated with mucus-secreting adenocarcinoma. Case report and discussion of the histogenesis. Pancreatology. 2005. 5:279–284.
7. Shiozawa M, Imada T, Ishiwa N, Rino Y, Hasuo K, Takanashi Y, et al. Osteoclast-like giant cell tumor of the pancreas. Int J Clin Oncol. 2002. 7:376–380.
8. Oehler U, Jürs M, Klöppel G, Helpap B. Osteoclast-like giant cell tumour of the pancreas presenting as a pseudocyst-like lesion. Virchows Arch. 1997. 431:215–218.
9. Gao L, Li ZS, Jin ZD, Man XH, Zhang MH, Zhu MH. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas diagnosed by endoscopic ultrasonography-guided fine-needle aspiration. Chin Med J (Engl). 2009. 122:1598–1600.
10. Goldberg RD, Michelassi F, Montag AG. Osteoclast-like giant cell tumor of the pancreas: immunophenotypic similarity to giant cell tumor of bone. Hum Pathol. 1991. 22:618–622.




 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download