Abstract
Intra-abdominal desmoplastic small round cell tumor (DSRCT) is a highly malignant tumor of uncertain histogenesis. Here we report a case of DSRCT involving the stomach, initially misdiagnosed as gastric cancer. A 12-year-old boy presented with upper abdominal pain developed 1 month prior. On gastroscopy, a 7-cm mass was noted involving the esophago-gastric junction to the fundus, and positron emission tomography showed multiple hot uptakes suggesting distant metastasis. Gastroscopic biopsy showed poorly differentiated malignant cells. We diagnosed as stage IV gastric cancer and treated with 6 cycles of chemotherapy. Laparotomy revealed a huge gastric mass along with peritoneal disseminations. Palliative proximal gastrectomy was performed. Pathological examination revealed transmural involvement of DSRCT, and t(11;22)(p12;q12) was demonstrated on fluorescence in situ hybridization test. The chemotherapeutic regimen was changed and the patient underwent 8 additional cycles of post-operative chemotherapy. The patient is now alive and the residual tumor shows no significant changes after chemotherapy.
Intraabdominal desmoplastic small round cell tumors (DSRCT) are rare, but aggressive mesenchymal malignancies of uncertain origin [1]. This tumor typically occurs during adolescence and young adult life in males. It usually presents with symptoms of abdominal pain, weight loss, nausea, vomiting, and a palpable abdominal mass [1]. Findings at laparotomy frequently include peritoneal dissemination of uncertain origin, ascites, and occasionally visceral implantations forming a mass [1]. Since primary gastrointestinal (GI) malignancies are rare in children, the mass-forming DSRCT mimicking a GI tract tumor is difficult to diagnose before a laparotomy. Here we report a case of DSRCT involving the stomach that was initially misdiagnosed as a gastric cancer.
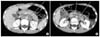
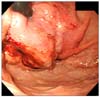
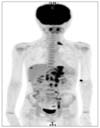
A 12-year-old boy presented with epigastric pain that developed 1 month ago. An episode of syncope with hematochezia was reported 2-week ago. The family and medical history were unremarkable. On physical examination, the patient appeared pale with no other significant findings noted. Laboratory tests revealed hemoglobin of 7.0 g/dL and no other abnormal findings. On gastroscopy, a 7-cm mass was noted at the esophago-gastric junction and posterior wall of the cardia. The overlying mucosa and the bridging folds appeared normal, thus suggesting a submucosal tumor (Fig. 1). Abdominal computed tomography (CT) showed a large gastric mass with multiple enlarged retropancreatic and aortocaval lymph nodes (Fig. 2). Positron emission tomography (PET) showed multiple uptake suggesting metastases to the left supraclavicular, right pleural, pelvic and retroperitoneal lymph nodes (Fig. 3). The gastroscopic biopsy specimen showed a poorly differentiated carcinoma.
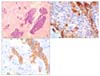
Based on the findings of malignant mass on gastroscopy and pathological examination, metastatic lesions on CT and PET, we diagnosed as the stage IV gastric cancer. The patient underwent 6 cycles of neo-adjuvant chemotherapy with docetaxel and cisplatin. Evaluation after the chemotherapy showed a slightly regressed gastric mass; however, the retroperitoneal metastases progressed. On laparotomy, a huge mass from the lesser curvature of the stomach invading the gastrohepatic ligament was noted along with numerous peritoneal implantations. The tail of pancreas was palpated hardly, suggesting invasion from the retropancreatic metastases. Palliative proximal gastrectomy with esophago-gastric anastomosis was performed, and the final pathological examination revealed transmural involvement of DSRCT (Fig. 4). A reciprocal translocation t(11;22)(p13;q12) was demonstrated on fluorescent in situ hybridization test. The post-operative chemotherapeutic regimen was changed to CCG 7881B: cyclophosphamide, doxorubicin, and vincristine. The patient underwent 8 cycles of CCG 7881B, but the residual disease showed no changes.
Intraabdominal DSRCT has rarely been reported in adolescents and young adult males, and few long-term survivors have been described and the survival prognosis following the diagnosis is an average of 23 months [2]. Due to the highly aggressive nature and diffuse spread of the DSRCT, systemic chemotherapy has been used as the initial treatment along with debulking surgery. Lal et al. [3] achieved an over 90% reduction of the tumor bulk in 71% of their patients and demonstrated a significant survival benefit, thus suggesting the benefit of aggressive surgical resection. By contrast, Livaditi et al. [4] reported that even radical surgical efforts were palliative with a dismal outcome despite all therapeutic modalities. It was impossible in the case reported here to achieve a large volume reduction at surgery because of the massive retroperitoneal involvement. Whether it is worth sacrificing major organs such as the liver and pancreas for cytoreductive surgery in patients with DSRCT is debatable.
Primary gastric cancer, although rare, has been reported sporadically in children and the reported incidence is about 0.1% [5]. However, DSRCT of the stomach is extremely rare; only one case has been previously reported in children [5]. The rarity of this condition along with the inconspicuous pathology led us to misdiagnose as stage IV gastric cancer before surgery. We suggest that, when poorly differentiated malignancies of the stomach are being considered, DSRCT should be in the differential diagnosis, especially among adolescent and young adult males. Moreover, since DSRCT can form a mass along the whole GI tract, thus mimicking a primary GI tract tumor, DSRCT should be included in the differential diagnosis of primary GI tract tumors in children.
DSRCT typically display clusters or nests of small round cells lying in a hypocellular, desmoplastic, collagenous stroma and this is a helpful diagnostic feature. In immunohistochemical staining, the DSRCT is usually positive for desmin (dot-like expression) and cytokeratin [6]. The case reported here satisfies the typical pathological and immunohistochemical staining profiles and we could make the diagnosis of DSRCT, which was impossible before laparotomy.
The reciprocal translocation t(11;22)(p13;q12) is unique to the DSRCT and confirms the diagnosis [7]. The translocation fuses two specific genes: the Ewing sarcoma gene (found in Ewing's sarcoma) and the Wilms tumor 1 gene (found in Wilms' tumor), and the resultant chimera protein is thought to act as a transcriptional regulator [8]. This transcriptional factor is known to regulate the expression of specific target genes, and the dysregulated expression of the target genes is related to the invasiveness of the DSRCT [9]. Recently, connective tissue growth factor (CCN2) that is highly expressed in DSRCT, has been found to regulate the tumor cell growth, matrigenesis, and angiogenesis of this tumor [10]. As conventional multimodal treatments fail to improve survival, future treatment should include specific targeted treatment focusing on these cellular regulatory mechanisms.
"Initially responsive but soon refractory to the treatment and rapidly progressive" depicts the clinical behavior of the DSRCT [4]. The case reported here showed an initial treatment-response of the gastric mass after the neoadjuvant chemotherapy; however, the residual tumors remained nearly unchanged postoperatively after adjuvant chemotherapy with CCG 7881B. The tumors are likely now in a refractory phase and will soon show rapid disease progression, eventually leading to the death of this patient.
In summary, a patient with intraabdominal DSRCT mimicking a gastric cancer is presented in this report. The diagnosis was determined after laparotomy. The diffuse intraabdominal spread made it impossible to extirpate the lesions completely. Although post-operative chemotherapy was provided and the patient is now alive with residual disease, the prognosis is dismal.
Figures and Tables
Fig. 2
Multiple, enlarged (A) retropancreatic and (B) aortocaval, retrocaval lymph nodes are seen on abdominal computed tomography scan (line-indicated).
References
1. Gerald WL, Miller HK, Battifora H, Miettinen M, Silva EG, Rosai J. Intra-abdominal desmoplastic small round-cell tumor. Report of 19 cases of a distinctive type of high-grade polyphenotypic malignancy affecting young individuals. Am J Surg Pathol. 1991. 15:499–513.
2. Frappaz D, Bouffet E, Dolbeau D, Bouvier R, Carrie C, Louis D, et al. Desmoplastic small round cell tumors of the abdomen. Cancer. 1994. 73:1753–1756.
3. Lal DR, Su WT, Wolden SL, Loh KC, Modak S, La Quaglia MP. Results of multimodal treatment for desmoplastic small round cell tumors. J Pediatr Surg. 2005. 40:251–255.
4. Livaditi E, Mavridis G, Soutis M, Papandreou E, Moschovi M, Papadakis V, et al. Diffuse intraabdominal desmoplastic small round cell tumor: a ten-year experience. Eur J Pediatr Surg. 2006. 16:423–427.
5. Murray JC, Minifee PK, Trautwein LM, Hicks MJ, Langston C, Morad AB. Intraabdominal desmoplastic small round cell tumor presenting as a gastric mural mass with hepatic metastases. J Pediatr Hematol Oncol. 1996. 18:289–292.
6. Lae ME, Roche PC, Jin L, Lloyd RV, Nascimento AG. Desmoplastic small round cell tumor: a clinicopathologic, immunohistochemical, and molecular study of 32 tumors. Am J Surg Pathol. 2002. 26:823–835.
7. Sawyer JR, Tryka AF, Lewis JM. A novel reciprocal chromosome translocation t(11;22)(p13;q12) in an intraabdominal desmoplastic small round-cell tumor. Am J Surg Pathol. 1992. 16:411–416.
8. Gerald WL, Ladanyi M, de Alava E, Cuatrecasas M, Kushner BH, LaQuaglia MP, et al. Clinical, pathologic, and molecular spectrum of tumors associated with t(11;22) (p13;q12): desmoplastic small round-cell tumor and its variants. J Clin Oncol. 1998. 16:3028–3036.
9. Gerald WL, Haber DA. The EWS-WT1 gene fusion in desmoplastic small round cell tumor. Semin Cancer Biol. 2005. 15:197–205.
10. Rachfal AW, Luquette MH, Brigstock DR. Expression of connective tissue growth factor (CCN2) in desmoplastic small round cell tumour. J Clin Pathol. 2004. 57:422–425.




 Citation
Citation Print
Print


 XML Download
XML Download