Abstract
Lymphangiomas are rare congenital benign tumors arising from the lymphatic system, and are mostly encountered in the neck and axillary regions of pediatric patients (95%). Lymphangioma of the pancreas is extremely rare accounting for less than 1% of these tumors. We report here on a case of pancreatic cystic lymphangioma. A 54-year-old woman presented with intermittent postprandial abdominal discomfort and radiating back pain. Abdominal computed tomography scan revealed 8 × 6.5 cm hypodense cystic mass arising from the tail of the pancreas without septa or solid component. The initial impression was a pancreatic pseudocyst. The patient underwent distal pancreatectomy with splenectomy. The histopathologic and immunohistochemical study helped make the diagnosis of a pancreatic cystic lymphangioma. Herein, we report a case of pancreatic cystic lymphangioma mimicking pancreatic pseudocyst and review the relevant medical literature.
Cystic lesions in the pancreas include pseudocysts, simple cysts, serous cystadenomas, mucinous cystic neoplasms and intraductal papillary mucinous neoplasms. Another rare cystic lesion is pancreatic lymphangioma. Lymphangiomas are rare, benign, and congenital malformations of the lymphatic system [1] and are mostly encountered in the neck (75%) and axillary regions (25%), though a variety of other sites have been described including the mediastinum, pleura, pericardium, groin, bones and abdomen [2]. Pancreatic lymphangioma is an extremely rare tumor accounting for less than 1% of these tumors. And since its first description by Koch in 1913, less than 100 such cases have currently been reported in the literature [3]. Here we report on a case of pancreatic cystic lymphangioma that mimicked pancreatic pseudocyst, and the tumor was diagnosed according to the results of the histopathological and immunohistochemical studies.
A 54-year-old woman presented with intermittent postprandial abdominal discomfort and radiating back pain that she had suffered during the past month. She had no trauma history, but several episodes of pancreatitis. On physical examination, a palpable mass without clear borders was detected on the left subchondral region. Laboratory findings, including serum carcinoembryonic antigen and carbohydrate antigen 19-9, were within normal limits. Abdominal computed tomography (CT) revealed the presence of a well-circumscribed homogenous mass (about 8 × 6.5 cm) arising from the tail of the pancreas without septa or enhancing solid portion (Fig. 1). The wall of the tumor was slightly enhanced after administering intravenous contrast medium. No regional lymphadenopathy, ascites or metastasis was demonstrated. From these radiologic findings, we diagnosed a pancreatic pseudocyst. At laparotomy, we identified a cystic mass of about 7 × 6.5 cm originating from the body of the pancreas, and a milky fluid was contained within the mass (Fig. 2). The patient underwent distal pancreatectomy with splenectomy. Microscopically, the specimen showed enlarged cyst-like space lined by endothelial cells contained within lymph-like fluid content (Fig. 3A). The endothelial cells lining the surface of the cystic spaces were positive for D2-40 (Fig. 3B). There was no staining of CD34, caletinin, or MOC-31. The final diagnosis was cystic lymphangioma of the pancreas. The patient had an uneventful recovery and she was discharged on the 18th postoperative day. There was no evidence of recurrence in the 6 months after surgery.
If lymphangioma arises within the pancreatic tissue or if it is attached to the pancreas with a pedicle, it is termed as pancreatic lymphangioma [3].
Cystic lymphangioma of the pancreas is usually a large lesion with an average diameter of 12.3 cm [4]. It can be seen in any age group but more frequent in females and is often located in the body and tail of the pancreas [2].
The etiology of lymphangiomas remains unclear; a well-established theory suggests that lymphangiomas arise from sequestrations of lymphatic tissue during embryologic development. However, it has been suggested that abdominal trauma, lymphatic obstruction, inflammatory process, surgery or radiation therapy may lead to the secondary formation of lymphangioma [5].
The clinical picture of pancreatic lymphangiomas is usually symptomatic (92.2%). Abdominal pain and palpable mass seem to be the most common symptoms [2]. Jaundice was reported in only one case [4]. Our patient presented with postprandial abdominal discomfort and radiating back pain. Furthermore, although rare, acute abdomen can occur due to complications such as intestinal obstruction, rupture and/or hemorrhage [6].
Abdominal ultrasonography, CT, magnetic resonance imaging (MRI), angiography and fine needle aspiration biopsy (FNAB) can be employed for the preoperative diagnosis of lymphangiomas. An ultrasound will show a complex cystic mass owing to internal septa. On CT, the tumor is a well-circumscribed, encapsulated, water-isodense, polycystic tumor with thin septa, similar in appearance to cystadenomas, which occur far more frequently [7]. Multiple fine septations and thin walls may enhance after IV contrast injection [1]. Although CT provides additional information about the characteristics of the lesions, the diagnosis is often not direct. It is often misdiagnosed as pseudocyst, or cystic neoplasm as in our case. Preoperative FNAB is still controversial as it might cause hemorrhage, rupture or tumor implantation in malignant cases [3]. MRI does not yield any further information [1]. Although various imaging features can help, definitive diagnosis requires pathologic examination of the excised lesion. In our case, abdominal CT revealed a homogenous cystic mass without septa or enhancing solid portion. Therefore, it was thought that the pancreatic mass mimicked pancreatic pseudocyst.
Microscopically, cystic lymphangiomas consists of dilated lymphatic channels of varying size divided by thin septae. The cystic wall is lined by thin and flat endothelial cells [8]. Immunohistochemically, the diagnosis of a pancreatic lymphangioma was supported immunohistochemically by positive staining of factor VIII-R antigen, CD31 and CD34, which are sensitive and specific markers for identification of lymphatic capillary endothelium [2]. According to Kahn et al. [9], D2-40 is a new selective marker of lymphatic endothelium. Therefore, immunohistochemical finding shows positive endothelial-lined cells in immunostaining by D2-40 in pancreatic lymphangioma, as in our case.
Surgical excision is the current treatment of choice [3], since incomplete resection may lead to recurrence [2]. Laparotomy is generally preferred; however, laparoscopic resection can also be performed in suitable cases. Total resection of the tumor is mandatory.
Although lymphangioma is a benign lesion, it often behaves in an aggressively invasive manner and can grow to an enormous size. Therefore, resection of adjacent organs may be required to accomplish complete excision.
In conclusion, although extremely rare, lymphangioma of the pancreas should be taken into consideration as a differential diagnosis of a pancreatic cystic lesion, especially in women.
Figures and Tables
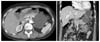 | Fig. 1Abdominal computed tomography (CT) findings. The abdominal CT scan reveals an approximately 8 × 6.5 cm-sized well-circumscribed homogenous cystic mass arising from the tail of the pancreas without septa or enhancing solid portion. |
References
1. Koenig TR, Loyer EM, Whitman GJ, Raymond AK, Charnsangavej C. Cystic lymphangioma of the pancreas. AJR Am J Roentgenol. 2001. 177:1090.
2. Igarashi A, Maruo Y, Ito T, Ohsawa K, Serizawa A, Yabe M, et al. Huge cystic lymphangioma of the pancreas: report of a case. Surg Today. 2001. 31:743–746.
3. Casadei R, Minni F, Selva S, Marrano N, Marrano D. Cystic lymphangioma of the pancreas: anatomoclinical, diagnostic and therapeutic considerations regarding three personal observations and review of the literature. Hepatogastroenterology. 2003. 50:1681–1686.
4. Lyngdoh TS, Konsam R, Th B, Marak B. Giant cystic lymphangioma of pancreas. ANZ J Surg. 2008. 78:673–674.
5. Schneider G, Seidel R, Altmeyer K, Remberger K, Pistorius G, Kramann B, et al. Lymphangioma of the pancreas and the duodenal wall: MR imaging findings. Eur Radiol. 2001. 11:2232–2235.
6. Itterbeek P, Vanclooster P, de Gheldere C. Cystic lymphangioma of the pancreas: an unusual cause of the acute surgical abdomen. Acta Chir Belg. 1997. 97:297–298.
7. Gray G, Fried K, Iraci J. Cystic lymphangioma of the pancreas: CT and pathologic findings. Abdom Imaging. 1998. 23:78–80.
8. Chung JC, Kim HC, Chu CW, Shin EJ, Lim CW, Song OP. Huge cystic lymphangioma of the pancreas. Can J Surg. 2009. 52:E303–E305.
9. Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol. 2002. 15:434–440.




 Citation
Citation Print
Print




 XML Download
XML Download