Abstract
A 50-year-old male, renal transplant recipient, was admitted with fever and chest discomfort. At admission, chest radiologic finding was negative and echocardiography showed minimal pericardial effusion. After 2 days of admission, chest pain worsened and blood pressure fell to 60/40 mmHg. Emergency echocardiography showed a large amount of pericardial effusion compressing the entire heart. Pericardiocentesis was performed immediately. Mycobacterium tuberculosis was isolated from pericardial fluid. Tuberculosis pericarditis should be considered as the cause of cardiac tamponade in renal transplant recipients, even with the absence of pericardial effusion in the initial study or suggestive history.
Mycobacterium tuberculosis infection affects less than 1% of solid organ transplant recipients, but cumulative incidence is three times that of the general condition [1,2]. Tuberculosis infection represents an important cause of mortality and morbidity in solid organ transplant recipients [3]. The higher incidence of extrapulmonary tuberculosis infection in renal transplant recipients can retard diagnosis and treatment with increased morbidity and mortality rates [3,4]. Here we report a case that cardiac tamponade caused by rapidly increasing pericardial effusion due to tuberculosis pericarditis.
A 50-year-old male was admitted to the hospital with two weeks history of fever and chest discomfort. There was no other complaint. He had deceased donor kidney transplantation one month ago for hypertension and chronic renal failure and was on tacrolimus 2.5 mg bid, methylprednisolone 8 mg bid, and mycophenolate mofetil 500 mg bid. Drug levels of tacrolimus and mycophenolic acid were 5.9 ng/mL (normal range, 5 to 20 ng/mL) and 1.89 mg/L (normal range, 1.0 to 4.0 mg/L). His general condition was tolerable and vital signs were blood pressure 118/69 mmHg, heart rate 91/min, respiration rate 20/min, and body temperature 37.1℃. Initial laboratory study were white blood cell counts 9,300/µL, neutrophil 8,810/µL, lymphocyte 90/µL, hemoglobin 7.5 g/dL, platelet counts 136,000/µL, blood urea nitrogen 67.6 mg/dL, creatinine 3.57 mg/dL, and C-reactive protein 7.83 mg/dL. Chest X-ray showed clear lung fields. Blood, urine, sputum, and stool culture were all negative. Chest computed tomography showed minimal multifocal patchy peribronchialitis. He received intravenous piperacillin-tazobactam 9 g per day for possible pneumonia. Echocardiography showed minimal amount of pericardial effusion and electrocardiogram showed normal sinus rhythm. Renal Doppler showed no demonstrable abnormality in the transplanted kidney.
Despite the use of antibiotics, he remained unwell with chest discomfort and chest pain except fever subsided. After 2 days of admission, chest pain got worsen and blood pressure fall to 90/60 mmHg. Electrocardiogram showed ST elevation in lead I, II and V4 to V6 and ST depression in aVR. Cardiac profiles showed Troponin I 0.018 ng/mL (normal range, 0 to 0.78 ng/mL), creatine kinase-MB 0.51 ng/mL (normal range, 0 to 5 ng/mL), N-terminal prohormone of brain natriuretic peptide 4,349 pg/mL (normal range, 0 to 88 pg/mL). Nitroglycerin test was positive. The chest pain was controlled by morphine. Coronary angiography was taken to rule out myocardial infarction. Coronary angiography showed no significant coronary artery disease and no collapsed chamber.
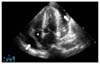
Under intensive care unit monitoring, his blood pressure gradually decreased and reached 60/40 mmHg. Emergency transthoracic echocardiography was performed. The parasternal long-axis and apical four-chamber views showed large pericardial effusion which was surrounding heart, reaching 3 to 4 cm thickness in some parts, and causing marked compression to the whole heart (Fig. 1). Pericardiocentesis was performed immediately and nearly 1 L of serous fluid was aspirated from the patient. Samples from the pericardial fluid were prepared for biochemical, microbiologic, and pathologic examinations. After pericardiocentesis, artery blood pressure began to increase, and chest pain subsided. The results of pericardial fluid were white blood cells>1,000/µL (neutrophil 90% and lymphocyte 1%), glucose 249 mg/dL, protein 3,890 mg/dL, lactate dehydrogenase 2,221 U/L, and albumin 2,020 mg/dL. Biochemical analysis showed exudative pericardial effusion. Acid-fast staining of pericardial fluid was positive, but the results for other sites such as sputum and urine were negative. Mycobacterium tuberculosis was isolated from pericardial fluid. Tacrolimus and mycophenolic mofetil except steroids were discontinued. He receive steroid (60 mg) for the treatment of acute pericarditis. Antituberculosis treatment with isoniazid 300 mg qd, rifampin 600 mg qd, ethamubutol 1,200 mg qd, and pyrazinadmide 1,500 mg qd was initiated. Symptoms such as chest pain and fever were subsided after the initiation of antituberculosis treatment. After 7 days, tacrolimus and mycophenolate mofetil were added for immunosuppression. The drug level of cyclosporine sustained low due to the drug interaction with rifampin, so we changed the drug to levofloxin 250 mg. The patient's general condition improved and creatinine decreased to 2.15 mg/dL. He was discharged from the hospital. During the follow-up period of 3 months, no sign of deterioration was observed and echocardiographic finding showed minimal pericardial effusion without hemodynamic instability.
Tuberculosis pericarditis is detected in 1 to 2% of all acute pericarditis cases. Cardiac tamponade is the main presentation in 7% of these cases [5]. Pre-operative purified protein derivative of tuberculin testing is routinely performed currently in kidney transplant recipients because Korea is tuberculosis-endemic area.
About 45 to 60% of tuberculosis occurs in the first year (median onset time, 9 months) after renal transplantation [6]. Cough, shortness of breath, orthopnea, night sweats, weight loss, and ankle edema are common symptoms in tuberculosis pericarditis. The most common findings are cardiomegaly, pericardial rub, fever, and tachycardia [7]. Our case showed fever, chest discomfort and pain, but other cardiac problems were not present. In our case, cardiac tamponade was rapidly developed by increased pericardial effusion with symptoms such as chest discomfort and pain, even though initial echocardiography showed minimal pericardial fluid. The pericardial tuberculosis infection was unexpected as there was no risk factor or history of tuberculosis and the radiologic findings of chest X-ray and chest computed tomography were clear.
Tuberculosis pericarditis should be considered as the cause of cardiac tamponade in renal transplant recipients, even with the absence of pericardial effusion in the initial study or suggestive history.
Figures and Tables
References
1. Alothman A, Al Abdulkareem A, Al Hemsi B, Issa S, Al Sarraj I, Masoud F. Isolated hepatic tuberculosis in a transplanted liver. Transpl Infect Dis. 2004. 6:84–86.
2. Hsu MS, Wang JL, Ko WJ, Lee PH, Chou NK, Wang SS, et al. Clinical features and outcome of tuberculosis in solid organ transplant recipients. Am J Med Sci. 2007. 334:106–110.
3. Subramanian AK, Nuermberger EL. Tuberculosis in transplant recipients: diagnostic and therapeutic dilemmas. Transpl Infect Dis. 2008. 10:229–230.
4. Muñoz P, Rodríguez C, Bouza E. Mycobacterium tuberculosis infection in recipients of solid organ transplants. Clin Infect Dis. 2005. 40:581–587.
5. Fowler NO. Tuberculous pericarditis. JAMA. 1991. 266:99–103.
6. Singh N, Paterson DL. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis. 1998. 27:1266–1277.
7. Trautner BW, Darouiche RO. Tuberculous pericarditis: optimal diagnosis and management. Clin Infect Dis. 2001. 33:954–961.




 Citation
Citation Print
Print



 XML Download
XML Download