Abstract
Graft-versus-host disease (GVHD) is a rare complication after kidney transplantation. We describe a 62-year-old female with end-stage renal disease due to hypertension. She received a kidney with 4 mismatched human leukocyte antigen (HLA) out of 6 HLA - A, B, DR from a deceased donor. After the procedure, the patient showed watery diarrhea on postoperative day (POD) 45. An endoscopic biopsy of the colon revealed some apoptotic cells consistent with GVHD. Thrombocytopenia was gradually developed on POD 54. She received steroid pulse therapy, and thrombocytopenia did not progress. However, pneumonia, renal failure, and cardiac failure occurred. She died due to multiple organ failure. We must consider GVHD in renal transplant recipients without homozygous or identical HLA, who had only watery diarrhea without other typical GVHD symptoms such as skin rash and fever, although GVHD is rare in renal transplant recipients.
Graft-versus-host disease (GVHD) is a lethal complication after solid organ transplantation [1]. Mortality rate was reported more than 75% [2]. The diagnosis is often delayed because the early signs such as skin rash, fever, diarrhea or liver dysfunction are mistaken for drug reactions or infections [3]. Later, the patients develop pancytopenia which is the most frequent cause of death [1].
A number of GVHD cases have been reported to occur in small bowel and liver transplant recipients, with incidence rate of 1 to 2% [4]. However, GVHD has rarely been reported as a complication of kidney transplantation. In the review of the literatures, GVHD after kidney transplantation has occurred in only three cases. We describe GVHD in renal transplant recipient who had 4 mismatched human leukocyte antigen (HLA) out of 6 HLA - A, B, DR from a deceased donor.
The patient was 62-year-old Korean female with end-stage renal disease due to hypertension requiring 3 years of peritoneal dialysis. She had received two HLA matched, deceased donor kidney transplant from 35-year-old male with pontine hemorrhage. The blood type of the patient was AB positive and she had no panel-reactive antibodies before the transplantation. Her HLA type was HLA-A: 24,33; B: 44,60; DR: 4,-. The donor was B and DR positive with HLA type of HLA-A: 2,11; B: 60,51; DR: 4,14. She received immunosuppressive agents with 1.5 mg/kg of antithymocyte globulin as induction therapy, and was maintained with tacrolimus, mycophenolate mofetil, and methylprednisolone by our usual protocol.
The creatinine serum level steadily decreased, but lowest creatinine level was 2.5 mg/dL on postoperative day (POD) 23. On POD 25, serum creatinine level abruptly increased to 3.0 mg/dL, and we performed renal biopsy. The pathologic result revealed no evidence of acute cellular rejection, but benign nephrosclerosis consistent with donor's nephropathy. She discharged on POD 33.
About two weeks later, she was admitted to our hospital for watery diarrhea, abdominal discomfort, general weakness, and poor oral intake. On admission (POD 45), blood pressure was 132/79 mmHg, pulse rate 82/min, body temperature 36.1℃, and respiratory rate 20/min. The physical examination revealed chronic ill looking appearance of the patient and dehydrated lip, tongue, and skin. Abnormal skin rash was not presented in the patient. Initial laboratory values showed a white blood cell count of 1,210/µL with 8.7% lymphocytes, hemoglobin 6.9 g/dL, platelet count 118,000/µL, total bilirubin 0.2 mg/dL (normal range, 0.2 to 1.5 mg/dL), blood urea nitrogen 34.1 mg/dL, serum creatinine 4.64 mg/dL, and C-reactive protein 0.03 mg/dL (normal range, <0.3 mg/dL). Mycophenolic mofetil was discontinued due to watery diarrhea and abdominal discomfort. She was conservatively managed with bowel rest and parenteral nutritional support.
She went through an evaluation for watery diarrhea. The cytotoxicity assay of her stool was negative for Clostridium difficile toxin and stool cultures and special stains for microorganisms were all negative. Cytomegalovirus (CMV) antigenemia assay was also negative. On POD 47, gastroduodenoscopy showed multiple erosive lesions and normal mucosa in the colon was shown by colonoscopy. The work-up failed to demonstrate an infectious etiology of the gastrointestinal symptoms. On POD 48, all biopsied tissue from colon demonstrated some apoptotic bodies which were consistent with GVHD on histologic examination (Fig. 1). At this time, tacrolimus was stopped and only methylprednisolone was used. On POD 54, thrombocytopenia was gradually developed. She received the steroid pulse therapy, and thrombocytopenia did not progress. On POD 62, she complained of dyspnea, so chest X-ray and chest computed tomography (CT) was performed. Chest CT showed multifocal patchy ground-glass opacities and peribronchial consolidation, which suggested pneumocystis pneumonia or cytomegalovirus pneumonia. On POD 63, the patient developed oliguria, and serum creatinine gradually increased. Continuous renal replacement therapy was used due to oliguria and metabolic acidosis. On POD 64, bronchoscopy showed no endobronchial lesions, and no positive findings detected in the brochoalveolar lavage. On POD 65, electrocardiogram showed ST elevation on lead I-IV. Troponin I was 9.674 ng/mL (normal range, <0.78 mg/dL) and CK-MB 77.52 ng/mL (normal range, <5 mg/dL). Cardiac enzyme was abruptly elevated and blood pressure decreased to 70/40 mmHg. Coronary angiography was preceded for evaluation of cardiac disease. Coronary angiography revealed no vascular stenosis and suggested stress-induced cardiomyopathy. The patient expired on POD 67 from multiple organ failure. Throughout her hospital course, she received transfusion several times with blood products which were either leukocyte reduced or irradiated.
GVHD is the consequence of an immunologic reaction of engrafted lymphoid cells against the tissues of the host [4]. GVHD is mainly associated with allogenic hematopoietic stem cell transplantation, and occurs much less frequently after transplantation of immunologically active solid organs such as liver and small intestine. Four cases of GVHD after kidney transplantation were reported including the present case.
Typical symptoms and signs of GVHD were skin rash, severe diarrhea, and the elevation of total bilirubin, but these findings were often attributed by drug reactions or infections. These may make the delay of GVHD diagnosis. These clinical presentations occur when donor T lymphocytes transferred with the graft are activated by alloantigens expressed by host antigen presenting cells, which initiate an immune response against recipient tissues such as skin, bone marrow, and gastrointestinal tract. Specific tests have been used in diagnosis of GVHD. One is detection of macrochimerism which was defined as more than 1% donor nucleated cells in the peripheral blood of recipient, and the other is single-tandem repeat (STR) DNA analysis which quantifies relative amounts of different DNA in a single tissue sample. We did not use these methods due to several limitations. Much lymphocyte tissue from the donor is required for the detection of macrochimerism, but we could not acquire lymphoid tissue due to donor being deceased and STR DNA analysis could not also be use in our hospital.
We tested donor-derived Y chromosome detection after the patient died. The serum of blood could not be separated since the sample was from the dead, so we examined DNA which was taken from endoscopic biopsy specimen. The amount of DNA was 10 ng/mL. We tested for amelogenin assay which shows two band peaks at 104 base pair (bp) and 110 bp in man (Fig. 2), meaning donor-derived Y chromosome [5,6]. However, the DNA of the recipient showed only 1 band peak at 104 bp in amelogenin gene assay. The test did not show the evidence of donor-derived Y chromosome in the recipient.
The diagnosis of GVHD is based on the characteristic clinical presentations, histological proof of target tissue damage and molecular manifestation of invasion of recipient target tissue by donor T cells. Our case showed the typical clinical manifestations and histologic confirmation of GVHD.
The characteristics of patients who developed GVHD after kidney transplantation were summarized in Table 1. Donor HLA homozygosity was known as a risk factor for GVHD. Donors' haplotype were homozygous in two cases and one case was one haplotype identical. Our case was 4/6 mismatch. Onset time of GVHD in our case was 48 days, which is shorter than that of other 2 cases despite more HLA mismatch. In addition, the typical signs of GVHD such as skin rash and fever were not presented in our case.
Usually, the outcome of GVHD is overwhelming sepsis, marrow aplasia, and multiple organ failure. Bacterial infections may be less common due to the use of empiric broad-spectrum antibiotics. Antifungal and anti-CMV prophylaxis may be indicated because fungi and CMV are the most frequent cause of death [7]. However, the incidence of mortality after kidney transplantation was 50% (2 cases) in the review of the literatures, including this case. The cause of mortality was multiple organ failure due to fungal infection or viral infection.
In conclusion, we must consider GVHD in the renal transplant recipients with not homozygous or identical HLA, who had only watery diarrhea without other typical GVHD symptoms such as skin rash and fever, although GVHD is rare in renal transplant recipients.
Figures and Tables
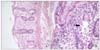 | Fig. 1Colonic biopsy specimen from patient shows acute graft versus host disease (GVHD). GVHD of the colon characterized rare intraepithelial lymphocytes in the absence of substantial inflammation, extensive crypt destruction in the lamina propria (A), and apoptotic cells (arrow) (B). |
References
1. Smith DM, Agura E, Netto G, Collins R, Levy M, Goldstein R, et al. Liver transplant-associated graft-versus-host disease. Transplantation. 2003. 75:118–126.
2. Burdick JF, Vogelsang GB, Smith WJ, Farmer ER, Bias WB, Kaufmann SH, et al. Severe graft-versus-host disease in a liver-transplant recipient. N Engl J Med. 1988. 318:689–691.
3. Smith DM, Agura ED, Levy MF, Melton LB, Domiati-Saad R, Klintmalm G. Graft vs host disease following kidney transplantation using an '0 HLA antigen mismatched' donor. Nephrol Dial Transplant. 2006. 21:2656–2659.
4. Sindhi R, Landmark J, Stratta RJ, Cushing K, Taylor RJ. Humoral graft-versus-host disease after pancreas transplantation with an ABO-compatible and Rh-nonidentical donor: case report and a rationale for preoperative screening. Transplantation. 1996. 61:1414–1416.
5. Nakahori Y, Takenaka O, Nakagome Y. A human X-Y homologous region encodes "amelogenin". Genomics. 1991. 9:264–269.
6. Pugatsch T, Oppenheim A, Slavin S. Improved single-step PCR assay for sex identification post-allogeneic sex-mismatched BMT. Bone Marrow Transplant. 1996. 17:273–275.
7. Gulbahce HE, Brown CA, Wick M, Segall M, Jessurun J. Graft-vs-host disease after solid organ transplant. Am J Clin Pathol. 2003. 119:568–573.
8. Ohtsuka Y, Sakemi T, Ichigi Y, Tanaka T, Nakamura K. A case of chronic graft-versus-host disease following living-related donor kidney transplantation. Nephron. 1998. 78:215–217.
9. Kato T, Yazawa K, Madono K, Saito J, Hosomi M, Itoh K. Acute graft-versus-host-disease in kidney transplantation: case report and review of literature. Transplant Proc. 2009. 41:3949–3952.




 Citation
Citation Print
Print





 XML Download
XML Download