Abstract
A case report described a 72-year-old man with a history of a deceased-donor liver transplantation (due to hepatitis B-associated end-stage liver cirrhosis) performed in 1994. The patient was diagnosed with renal cell carcinoma and pulmonary metastasis in 1997 and was successfully treated with radiofrequency ablation and thoracoscopic superior segmentectomy. There was no evidence of newly diagnosed metastatic lesions or recurrence until the 19th post-operative month. Gastric cancer was identified by endoscopy during a routine follow-up examination; the pre-pyloric antral lesion measured 1.5 cm in size and was histologically well-differentiated and confined to the submucosal layers on endoscopic ultrasound. Laparoscopic gastrectomy and lymph node dissection (D1 + β) was successfully performed in March 2009, and the patient was discharged on the 5th post-operative day without complications. This suggests that laparoscopic surgery is one of the feasible methods for resection of gastric cancer in liver transplant patients.
The development of novel and effective immunosuppressive agents has resulted in decreased graft rejection and improved graft or patient survival. There has been an increase in the incidence of post-transplantation de novo cancer; this occurred after the widespread use of anti-rejection drugs. Previous studies have emphasized the necessity for frequent clinical follow-ups for early cancer detection in transplant patients [1]. Minimally invasive surgery for surgical oncology is still controversial despite its popularity. Advancement of laparoscopic techniques and improvement in instrumentation have occurred since the initial introduction of laparoscopic cholecystectomy. Recent clinical trials have evaluated the safety and advantages of both laparoscopic and conventional open surgeries, and laparoscopic surgery is feasible in cancer treatment [2]. To our knowledge, there are no case reports evaluating laparoscopic gastric cancer surgery after liver transplantation. We describe a laparoscopic-assisted gastrectomy procedure for post-transplantation gastric cancer in the present study.
The patient was a 72-year-old man who had undergone a deceased-donor liver transplant for hepatitis B-associated end-stage liver cirrhosis in 1994 in a foreign country. Renal cell carcinoma with pulmonary metastasis was diagnosed in 1997 at our hospital and was successfully treated with radiofrequency ablation and thoracoscopic superior segmentectomy. There were no new metastatic lesions or recurrence for 19 months post-operatively; however, gastric cancer was incidentally diagnosed under endoscopy during the regular follow-up period. The lesion was located in the pre-pyloric antrum and measured 1.5 cm (Fig. 1); it was histologically well-differentiated adenocarcinoma and was confined to the submucosa on endoscopic ultrasound (Fig. 2).
The patient had other co-morbidities including hypertension and hyperlipidemia, and his current drugs included the immunosuppressive drug FK506 (Tacrolimus; Fujisawa Pharmaceutical Co., Osaka, Japan) (2 mg) once per day without additional steroids and hepatitis B immunoglobulin (Hepabig, Green Cross Co., Yongin, Korea) once per month. Pre-operative computed tomography revealed a replaced common hepatic artery which branched from the superior mesenteric artery (Fig. 3); this suggested that the recipient common hepatic artery was anastomosed to the replaced common hepatic artery at the site of the liver transplant. Pre-operative liver function tests were within normal limits.
The laparoscopic gastrectomy and lymph node dissection (D1 + β) procedures were performed in March 2009 (Fig. 4). The patient was placed in a supine position under general anesthesia. A traditional chevron incision had been used for the previous liver transplantation and a right paramedian incision had been used for a previous open cholecystectomy. For the laparoscopic procedure, an 11-mm trocar was carefully inserted through an umbilical incision for the laparoscopic scope using the open technique; an additional two 5 mm trocars was inserted through the left upper and lower abdominal quadrants. Severe upper abdominal adhesions were present, particularly between the liver and duodenum, but there was no evidence of metastatic lesions upon laparoscopic examination. The adhesions were carefully dissected, and trocars (5 mm and 12 mm) were inserted into the right abdominal wall (Fig. 5). We maintained a CO2 pneumoperitoneum below 12 mmHg secondary to hemodynamic instability due to old age and underlying disease. Previous antecolic Roux-en-Y hepaticojejunostomy and jejunojejunostomy sites were located (Fig. 6). The harmonic scalpel (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA) was primarily used for dissection of adhesions surrounding the liver and lymph nodes, and tissue manipulation near hepatic-associated vessels was minimized during lymph node dissection due to anatomical change after liver transplantation. The trocar incision was extended 5 to 6 cm transversely at the epigastrium after the intracorporeal procedure was completed; specimen removal and the Roux-en-Y gastrojejunostomy reconstruction proximal to the afferent loop jejunojejunostomy site was performed through this incision (Fig. 7). Surgical time was 300 minutes, and the estimated blood loss was 300 mL.
Fortunately, the final pathologic result was tumor confined to the mucosa without lymph node metastasis. Tacrolimus level was measured daily monitoring acute post-operative rejection; pre-operative levels measured at 2.7 ng/mL and dropped to 1.5 ng/mL on the 3rd post-operative day. However, there were no signs of acute rejection. Other liver function tests including alkaline phosphatase, bilirubin were within normal range during post-operative period. The patient re-started tacrolimus with soft diet on the 3rd post-operative day, and was discharged on the 5th post-operative day without complications. To our knowledge, the patient is in good general health and has suffered no post-operative recurrence.
The incidence of de novo cancer in transplant patients has been reported from 5 to 15% [3]. De novo cancer and age-related cardiovascular complications are two leading causes of late mortality in liver transplant recipients. Although the proportion of gastrointestinal de novo cancer is small, its development results in significant reductions in survival. The selection of appropriate patient treatment as well as early detection of de novo cancer through close follow-up is important for improvements in long-term survival [2].
Most patients have been treated by conventional open surgery secondary to concerns about adhesions and oncological safety. Laparoscopic surgery may have post-operative benefits over conventional open surgery due to reduced pain, faster return of bowel function, earlier oral feeding, and shorter hospital stays. However, there were no significant differences in short-term morbidity and mortality [4]. Several preliminary reports have suggested that laparoscopic surgery for early gastric cancer is feasible in terms of the oncological safety, although it must be confirmed through ongoing prospective randomized controlled trials [5]. We think this was not easy operation, but in our situation was possible because operator had a lot of laparoscopy-assisted distal gastrectomy experiences almost above 1,000 cases. Additionally, Adachi et al. [6] emphasized the advantages of laparoscopic surgery, including the minimization of tissue injury and the suppression of immune function [6]. Laparoscopic surgery resulted in fewer inflammatory and immunologic reactions compared to conventional open surgery in another study [7]. Management of transplant patients involves a balance between increasing doses of immunosuppressive agents (to prevent acute rejection) and decreasing doses (to suppress potential tumor activity). It is extremely important to minimize the extent of fluctuation of immunologic activities during the operative period for the prevention of acute rejection in the immunosuppressed patient.
The current treatment modality for early gastric cancer is determined by the presence of lymph node metastases in accordance with guidelines of the Japanese Research Society for Gastric Cancer [8]. Surgeons favor open surgical curative procedures with lymph node dissections rather than endoscopic repairs for submucosal lesions because the probability of lymph node metastasis ranged from 5 to 20% [9]. This patient was diagnosed with a submucosal lesion by pre-operative endoscopic ultrasound, and there was no evidence of recurrence or newly diagnosed metastasis during 19 months post-operatively. Therefore, we elected to perform laparoscopic curative surgery. However, endoscopic submucosal dissection and endoscopic mucosal resection could be considered alternative treatment options for early gastric cancer confined to mucosa without evidence of lymph node metastasis before deciding to undergo gastrectomy surgery.
Laparoscopic gastrectomy for early gastric cancer has been reported as a feasible minimally invasive surgical option in elderly patients with comorbidities [10]. However, special precautions are required to address the cardiopulmonary effects of pneumoperitoneum, systemic CO2 absorption, and anatomical changes resulting from previous transplant surgeries. Lymph node dissection near the portal veins and hepatic artery must be very carefully performed due to adhesions adjacent to the lymph nodes with accurate anatomical information, particularly in liver transplant patients. Accordingly, less invasive treatment methods are suitable for sicker patients. The patient in the present study underwent a more minimally invasive treatment course due to co-morbidities and advanced age, and successful treatment included radiofrequency ablation, thoracoscopic surgery, and laparoscopic gastrectomy. To our knowledge, this is the first case report to successfully use laparoscopic surgery to treat gastric cancer in liver transplant recipient patients. This data suggests that laparoscopic surgery is feasible for transplant patients if patients were appropriately selected.
Figures and Tables
Fig. 1
Endoscopic finding after performing biopsies shows early gastric cancer, type IIa + IIc lesion on lesser curvature of the gastric antrum.
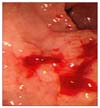
Fig. 3
Pre-surgical computed tomography angiography demonstrated the replaced common hepatic artery originating from the superior mesenteric artery. The left gastric artery and the splenic artery originate separately from the celiac axis and the common hepatic artery. A, aorta; White short arrow, proper hepatic artery; White long arrow, replaced common hepatic artery; Black short arrow, gastroduodenal artery; Black long arrow, left gastric artery; Arrowheads, superior mesenteric artery; Curved arrow, splenic artery.

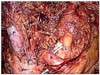
Fig. 4
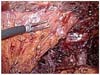
Intra-operative view after dissection of the D1+β lymph node around the celiac axis. P, portal vein; L, divided left gastric artery; S, splenic artery; PH, pancreatic head.
References
1. Herrero JI, Alegre F, Quiroga J, Pardo F, Iñarrairaegui M, Sangro B, et al. Usefulness of a program of neoplasia surveillance in liver transplantation: a preliminary report. Clin Transplant. 2009. 23:532–536.
2. Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, et al. Colon Cancer Laparoscopic or Open Resection Study Group. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009. 10:44–52.
3. Valero JM, Rubio E, Moreno JM, Pons F, Sanchez-Turrion V, Cuervas-Mons V. De novo malignancies in liver transplantation. Transplant Proc. 2003. 35:709–711.
4. Kim MC, Jung GJ, Kim HH. Morbidity and mortality of laparoscopy-assisted gastrectomy with extraperigastric lymph node dissection for gastric cancer. Dig Dis Sci. 2007. 52:543–548.
5. Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005. 241:232–237.
6. Adachi Y, Shiraishi N, Shiromizu A, Bandoh T, Aramaki M, Kitano S. Laparoscopy-assisted Billroth I gastrectomy compared with conventional open gastrectomy. Arch Surg. 2000. 135:806–810.
7. Jung IK, Kim MC, Kim KH, Kwak JY, Jung GJ, Kim HH. Cellular and peritoneal immune response after radical laparoscopy-assisted and open gastrectomy for gastric cancer. J Surg Oncol. 2008. 98:54–59.
8. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma - 2nd English edition. Gastric Cancer. 1998. 1:10–24.
9. An JY, Baik YH, Choi MG, Noh JH, Sohn TS, Kim S. Predictive factors for lymph node metastasis in early gastric cancer with submucosal invasion: analysis of a single institutional experience. Ann Surg. 2007. 246:749–753.
10. Hwang SH, Park do J, Jee YS, Kim HH, Lee HJ, Yang HK, et al. Risk factors for operative complications in elderly patients during laparoscopy-assisted gastrectomy. J Am Coll Surg. 2009. 208:186–192.




 Citation
Citation Print
Print


 XML Download
XML Download