Abstract
Hepatoid adenocarcinoma (HAC) is a tumor with aberrant hepatocellular differentiation that occurs in extrahepatic organs. HAC of the gallbladder is rare, and cases of alpha-fetoprotein production are extremely rare. A 61-year-old man was diagnosed with gallbladder adenocarcinoma after laparoscopic cholecystectomy. A radical operation including resection of liver bed and lymph node dissection was performed, and no tumor cell was found. However, at postoperative 19 months, he showed lymphadenopathy of the portocaval area and tumor thrombi in the right portal vein with high levels of serum alpha-fetoprotein. After right hemihepatectomy and portahepatis lymph node dissection was performed, he was diagnosed with metastatic HAC. On reviewing the gallbladder specimen, the tumor finally demonstrated HAC as the primary origin. Despite adjuvant therapy, the patient died from multiple liver metastasis 26 months after cholecystectomy. Although HAC of the gallbladder is a very rare malignancy, awareness of its existence is critical to avoid misdiagnosis.
Hepatoid adenocarcinoma (HAC) is a rare variant of adenocarcinoma, characterized by hepatic differentiation in both morphological and functional terms [1]. HAC occurs in various organs, most frequently in the stomach. Vardaman and Albores-Saavedra [2] described the first well-documented case of HAC of the gallbladder, and subsequently, to our knowledge, only 8 cases have been reported in the English literature [3-7]. However, some of these reports also had a poor description of the postoperative course. We report a very rare case of HAC from gallbladder with alpha-fetoprotein (AFP) production. Our report includes immunohistochemical features, the clinical course of poor prognosis through several operations with adjuvant therapy, and over a relatively long-term follow-up.
A 61-year-old man was referred to our hospital for further treatment of gallbladder cancer. Prior to presentation at our hospital, laparoscopic cholecystectomy for a gallbladder mass had been performed at a local hospital. Preoperative routine laboratory tests were within normal limits. However, the level of serum AFP was markedly elevated at 173.6 ng/mL (normal < 7.0). Carcinoembryonic antigen and carbohydrate antigen 19-9 were 2.0 ng/mL (normal < 5.0) and 10.3 U/mL (normal < 39), respectively. According to the pathology report from the local hospital, the lumen of gallbladder was filled with a white-gray necrotic mass, measuring 6 × 3 × 3 cm. The fundus of the gallbladder revealed a mass stalk of 2 × 2 cm. There was also a shallow ulcer measuring 2 × 1.5 cm. The resection margin of the cystic duct was tumor-free. Microscopic findings showed adenocarcinoma with moderate to poor differentiation. The 2 × 1.5 × 1 cm tumor was polypoid, and the carcinoma invaded the subserosal connective tissue. Vascular invasion was present, but no perineural or lymphatic invasion was seen. The patient was referred by advanced stage of gallbladder malignancy. A month after cholecystectomy, a radical operation including resection of liver bed and lymph node dissection was performed. On microscopy, the parenchyma around the liver bed demonstrated chronic granulomatous inflammation, and lymph nodes around the common hepatic artery and celiac trunk were negative. At 14 days after radical operation, the level of AFP had dropped to 10.1 ng/mL.
At 19 months after radical cholecystectomy, abdominal computed tomography (CT) showed lymphadenopathy of the portocaval area and tumor thrombi of the right portal vein (Fig. 1A). AFP was greatly elevated to 967.5 ng/mL. Positron emission tomography-CT revealed the hypermetabolic mass in the portocaval area, suggesting metastatic lymphadenopathy (Fig. 1B). Right hemihepatectomy with lymph node dissection was performed (Fig. 1C). The liver and lymph nodes of portocaval and inferior vena cava showed metastatic adenocarcinoma with hepatoid features (Fig. 2A). In immunohistochemical study, AFP and alpha-1 antitrypsin were positive in the hepatoid area and negative in the glandular area (Fig. 2B, C). Hepatocyte Paraffin 1 (Hep Par1), cytokeratin 7, and cytokeratin 20 were all negative. Slides from the previously operated gallbladder mass were reviewed to clarify the primary origin of metastatic HAC. Our results, combined with immunohistochemical analysis of previous operated gallbladder mass (Fig. 3), confirmed gallbladder as the tumor origin.
The patient received adjuvant chemotherapy (Gemcitabine and 5-fluorouracil) and radiotherapy (4,500 cGy, 5 weeks). However, 4 months after hepatectomy, he was readmitted with abdominal distension from ascites. Follow-up CT showed multiple metastases in the left lobe of liver and superior mesenteric vein thrombosis with extension into the portal vein. He died one month after readmission.
HAC is a special type of extrahepatic adenocarcinoma, with a striking morphologic similarity to hepatocellular carcinoma (HCC). HAC occurs predominantly in older patients, and is characterized by a very aggressive course, and a poor prognosis. Its biological behavior reflects extensive hematogenous metastasis to the liver, and the early and frequent involvement of lymph nodes is an important feature [8].
Hepatoid differentiation manifests as large polygonal cells with abundant clear and eosinophilic cytoplasm that are arranged in a trabecular, nested, or rosettoid pattern. The hepatoid nature of the clear tumor cells is demonstrated by bile production and immuno-phenotype [6]. Immunohistochemically, liver specific proteins, such as AFP and alpha-1 antitrypsin, are identified in the tumor cell. In the case presented here, the hepatoid area showed obvious positive immunoreactivity for AFP and alpha-1 antitrypsin, with negative glandular area. AFP is particularly important for diagnosis because it is usually positive in the tumors and elevated in the serum. However, HAC does not always produce AFP [3-5]. Although AFP positivity is important, it is not essential for a diagnosis of HAC. The diagnosis of HAC should be made on the basis of histopathological features of the tumor including immunohistochemical analysis, because the behavior of HAC is similar whether it produces AFP or not [3]. Hep Par1 has been used as a marker of hepatic differentiation, but subsequent studies have refuted its specificity and sensitivity [9,10]. In this case, Hep Par 1 was negative, and further studies are needed to determine its significance in diagnosis of HAC.
The main differential diagnosis for HAC of the gallbladder is HCC or combined HCC/cholangiocarcinoma invading the gallbladder. Van den Bos et al. [7] suggest the usefulness of magnetic resonance imaging as a problem-solving tool for analysis of rare tumors of non-hepatocellualr origin, including HAC of the gallbladder. In contrast to HCC or cholangiocarcinoma, HAC is characterized by high signal intensity in the periphery of the gallbladder lesion on T2 weighted images and slow peripheral enhancement without capsule enhancement or washout.
A review of previous reports [2-7] with HAC of the gallbladder found liver or lymph nodes metastasis in three cases. The other cases were limited by lack a description of metastasis or recurrence, and a relatively short postoperative follow-up period (8 to 15 months). In general, as seen in this case, HAC has a poor prognosis with frequent liver and lymph nodes metastasis.
Taken together, tumor location, radiologic features and high AFP are clues for diagnosis of HAC of the gallbladder. Although this is a very rare malignancy, awareness of its existence is critical to avoid misdiagnosis, especially since it is associated with a poor prognosis. Therefore, we suggest that aggressively radical resection, with the aim of complete tumor removal and cure, is the treatment of choice. In addition, close follow-up with frequent AFP check and abdominal imaging is recommended.
Figures and Tables
Fig. 1
(A) Abdominal computed tomography (CT) revealed the necrotic lymphadenopathy with infiltration of the portocaval area and thrombosis in the main and right portal vein. (B1 and B2) Positron emission tomography/CT showed the hypermetabolic lesion of 4 × 3 cm in portocaval area. (C) The cut surface of liver showed a well demarcated mass with a central bright yellowish area in the right portal vein.
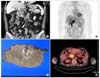
Fig. 2
(A) Highly pleomorphic cells arranged in a rosettoid, nested, or trabecular pattern with relatively clear cytoplasm, enlarged nuclei, and prominent nucleoli. Numerous hyaline globules are noted in the intercellular or intracellular areas (H&E ×200). Immunohistochemical staining for alpha-fetoprotein (B) and alpha1-antitrypsin (C) showed strongly positive in the hepatoid area of the liver (×200).

Fig. 3
(A) The gallbladder wall is replaced with infiltrating hepatoid adenocarcinoma composed of solid area, hepatoid carcinoma, with hyaline globules and conventional adenocarcinomatous area (H&E, ×200). Immunohistochemical staining for alpha-fetoprotein (B) and alpha1-antitrypsin (C) was positive in the hepatoid area of the previously operated gallbladder specimen (×200).

References
1. Supriatna Y, Kishimoto T, Uno T, Nagai Y, Ishikura H. Evidence for hepatocellular differentiation in alpha-fetoprotein-negative gastric adenocarcinoma with hepatoid morphology: a study with in situ hybridisation for albumin mRNA. Pathology. 2005. 37:211–215.
2. Vardaman C, Albores-Saavedra J. Clear cell carcinomas of the gallbladder and extrahepatic bile ducts. Am J Surg Pathol. 1995. 19:91–99.
3. Nakashima H, Nagafuchi K, Satoh H, Takeda K, Yamasaki T, Yonemasu H, et al. Hepatoid adenocarcinoma of the gallbladder. J Hepatobiliary Pancreat Surg. 2000. 7:226–230.
4. Sakamoto K, Monobe Y, Kouno M, Moriya T, Sasano H. Hepatoid adenocarcinoma of the gallbladder: case report and review of the literature. Pathol Int. 2004. 54:52–56.
5. Sakamoto K, Kimura N, Tokumura H, Ogasawara T, Moriya T, Sasano H. Hepatoid adenocarcinoma of the gallbladder. Histopathology. 2005. 47:649–651.
6. Gakiopoulou H, Givalos N, Liapis G, Agrogiannis G, Patsouris E, Delladetsima I. Hepatoid adenocarcinoma of the gallbladder. Dig Dis Sci. 2007. 52:3358–3362.
7. van den Bos IC, Hussain SM, Dwarkasing RS, Stoop H, Zondervan PE, Krestin GP, et al. Hepatoid adenocarcinoma of the gallbladder: a mimicker of hepatocellular carcinoma. Br J Radiol. 2007. 80:e317–e320.
8. Ishikura H, Kishimoto T, Andachi H, Kakuta Y, Yoshiki T. Gastrointestinal hepatoid adenocarcinoma: venous permeation and mimicry of hepatocellular carcinoma, a report of four cases. Histopathology. 1997. 31:47–54.
9. Maitra A, Murakata LA, Albores-Saavedra J. Immunoreactivity for hepatocyte paraffin 1 antibody in hepatoid adenocarcinomas of the gastrointestinal tract. Am J Clin Pathol. 2001. 115:689–694.
10. Terracciano LM, Glatz K, Mhawech P, Vasei M, Lehmann FS, Vecchione R, et al. Hepatoid adenocarcinoma with liver metastasis mimicking hepatocellular carcinoma: an immunohistochemical and molecular study of eight cases. Am J Surg Pathol. 2003. 27:1302–1312.




 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download