Abstract
Purpose
Acute respiratory failure is a relatively common complication in surgical patients, especially after abdominal surgery. Non-invasive ventilation (NIV) is increasingly used in the treatment of acute respiratory failure. We have assessed the usefulness of NIV in surgical patients with acute respiratory failure.
Methods
We retrospectively reviewed the medical charts of patients who were admitted to a surgical intensive care unit between March 2007 and February 2008 with acute respiratory failure. The patients who have got respiratory care for secondary reason such as sepsis and encephalopathy were excluded from this study.
Results
Of the 74 patients who were treated with mechanical ventilation, 15 underwent NIV and 59 underwent invasive ventilation. The causes of acute respiratory failure in the NIV group were atelectasis in 5 patients, pneumonia in 5, acute lung injury in 4, and pulmonary edema in 1, this group included 3 patients with acute respiratory failure after extubation. Overall success rate of NIV was 66.7%.
Acute respiratory failure (ARF) is a complication in surgical patients, especially after abdominal surgery. ARF after abdominal surgery is closely associated with a reduction in lung volume caused by alveolar collapse and dysfunction of the diaphragm, increased intraabdominal pressure after surgery, and decreased respiration caused by pain [1-3]. Treatment of post-operative ARF usually includes endotracheal tube insertion and mechanical ventilation.
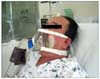
More recently, however, patients with post-operative ARF have been treated by non-invasive ventilation (NIV) [4], consisting of attachment of a mask to the face without endotracheal tube insertion (Fig. 1), followed by deliveries of positive air pressure by pressure support ventilation (PSV) and positive end expiratory pressure (PEEP) or continuous positive airway pressure (CPAP) [5,6]. NIV with positive pressure ventilation can relieve a patient's inspiratory efforts and improve gas exchange without the need for endotracheal intubation. This may benefit patients since endotracheal intubation has been associated with numerous complications. NIV decreases the development of ventilator associated pneumonia, allows the patient to eat and drink while receiving positive pressure ventilation and decreases the use of sedative drugs and neuromuscular blockers required to endure mechanical ventilation. Consequently, NIV shortens weaning time from mechanical ventilators and can avoid hypotension caused by the use of sedatives [5].
NIV was initially applied to treat patients with chronic obstructive pulmonary disease. In addition to successfully treating ARF, NIV reduced invasive intubation and mortality rates, and shortened treatment periods [7-9]. NIV has been utilized primarily to treat patients with hypercarbic respiratory failure, and its therapeutic role in treating psychogenic ARF and ARF after abdominal or thoracic surgery is expanding [10-14]. We therefore assessed the usefulness of NIV in surgical patients with ARF.
We retrospectively reviewed the medical records of the medical charts of patients admitted to the surgical intensive care unit in our institution for ARF between March 2007 and February 2008.
ARF was defined as a tachypnea (respiration rate >35), hypercapnea (PaCO2 >45 mmHg and PH <7.35), hypoxemia (PaO2/FiO2 <150), severe dyspnea, and the use of accessory respiratory muscles. Patients were excluded if they had hemodynamic instability, decreased mental status, inability to expectorate airway secretions or facial trauma.
NIV was performed using Performa Trak facial masks (Philips Respironics, Woerden, Netherland) either pressure control ventilation or PSV. Treatment goals included a volume per respiration of 6 to 8 mL/kg, with PEEP adjusted to 5 to 15 cmH2O according to the conditions of each patient's lung. Arterial blood gas analysis (ABGA), respiration rate and patient compliance were monitored continuously. NIV was converted to invasive ventilation with endotracheal intubation if respiratory status was worsened despite NIV support.
Weaning was performed using the optimal method that reduced the time of mechanical ventilation, including alternation of mechanical ventilation with venturi masks, reservoir bags. Patients were extubated if they were conscious, could breathe on their own, could readily expectorate, and could maintain smooth tidal volume under a positive pressure respiration <8 cm H2O.
ARF patients were divided into two groups, according to whether they underwent NIV or invasive ventilation. Their clinical features were compared including patient age, gender, acute physiology and chronic health evaluation (APACHE) II score at the time of the application of mechanical ventilation, PaO2/FiO2 ratio, whether or not immune suppressors were used, and the cause of respiratory failure. Causes of respiratory failure were diagnosed by clinical symptoms, ABGA, and chest radiographs. Success of treatment was defined as weaning from mechanical ventilation. Multivariate logistic regression analysis was used to determine factors predictive of the success of NIV.
We identified 74 patients who were treated in the surgical intensive care unit for ARF, including 45 patients who developed ARF immediately after elective surgery including transplantation, 17 patients taking immune suppressors after transplantation, 6 patients with multiple traumas, 4 patients who developed ARF immediately after emergency surgery, and 2 patients who developed ARF during follow-up observation after surgery other than transplantation. Of these 74 patients, 52 (77%) developed acute respiratory failure within 1 month after abdominal surgery or multiple traumas, with ARF directly associated with surgery and trauma. The 17 patients who developed ARF while taking immune suppressors were being treated with the latter after organ transplantation, including liver, kidney, pancreas, and other organs (Table 1).
Of the 74 ARF patients, 59 patients (79.7%) received invasive ventilation, and 15 (20.3%) received NIV (Table 2). Mean age in the invasive ventilation and NIV groups were 54 ± 18 years and 56 ± 14 years, respectively. The invasive ventilation group consisted of 39 males (66.2%) and 29 females (33.8%), whereas the NIV group consisted of 5 males (33.3%) and 10 females (66.7%). Causes of ARF in the invasive ventilation group were pneumonia 36 patients (61.0%), acute lung injury 14 patients (23.7%), atelectasis 3 patients (5.0%), pulmonary edema 3 patients (5.0%), and pulmonary embolism 1 patient (1.7%). Causes of ARF in the NIV group were atelectasis 5 patients (33.3%), pneumonia 5 patients (33.3%), acute lung injury 4 patients (26.7%), and patient with psychogenic pulmonary edema 1 patient (6.6%).
Of the 59 patients in the invasive ventilation group, 26 (44.1%) were immunosuppressed. Their mean APACHE II score at the time of ARF development was 17 ± 6 points, and their mean PaO2/FiO2 was 137± 69. Of the 15 patients in the NIV group, 5 (33.3%) were immunosuppresed. Their mean APACHE II score was 14 ± 6 points, and their mean PaO2/FiO2 was 115 ± 71 points.
The mean duration of artificial respirator application in the invasive ventilation group was 13.9 ± 18.0 days, their mean stay in the intensive care unit was 17.6 ± 19.6 days, and 13 of these 59 patients (22.0%) died. In comparison, the mean duration of artificial respirator application in the NIV group was 6.0 ± 6.0 days, their mean stay in the intensive care unit was 9.4 ± 7.6 days, and 2 of these 15 patients (13.3%) died.
Although the mean days of mechanical ventilation and stay in the intensive care unit differed in the two group, these differences were not statistically significant (P = 0.62 and 0.58 respectively). This may have been due to the severity of illness in several patients who received invasive ventilation requiring treatment for a long time.
Among the 15 patients in the NIV group, 10 (66.7%) were treated successfully without endotracheal intubation, whereas 5 (33.3%) were converted to invasive ventilation during treatment. The causes of conversion included patient noncooperation in 2 patients, the deterioration of hypercapnea in 1, deterioration of hypoxia in 1, and dyspnea symptoms in 1 (Table 3). Causative diseases in these 5 patients included atelectasis in 2, pneumonia in 2 and psychogenic pulmonary edema in 1. The mean time to conversion in these 5 patients was 28.2 hours after the start of NIV. Particularly, in patients whose causative disease was pneumonia, there was a trend toward a longer treatment period even after the conversion to invasive ventilation.
Of the 59 patients who received invasive ventilation, 3 were treated by NIV for respiratory failure after extubation, thus avoiding re-intubation (Table 4). Respiratory failure after extubation was defined as development of dyspnea symptoms within 12 hours after weaning. Of these 3 patients, 2 had post-operative atelectasis and 1 had acute lung injury due to multiple trauma. All were treated successfully by NIV without re-intubation.
NIV was successful in 10 of 15 patients. Univariate and multivariate analysis showed that success was related to the etiology of ARF, especially atelectasis (P = 0.032). NIV was more likely to be successful in patients with post-operative atelectasis, whereas invasive ventilation was more likely to be successful in patients with pneumonia. In contrast, patient age, gender, treatment with or immune suppressors, and the severity of respiratory function were not predictive of success (Table 2).
We have shown here that surgical patients with ARF can be treated effectively with NIV. The likelihood of NIV success is determined by the disease causing ARF not by the severity at the time of ARF or level of hypoxia. In particular, NIV was more likely to be successful in patients with postoperative atelectasis, whereas invasive ventilation was more likely to be successful in patients with pneumonia.
Although, hypoxia develops in 30 to 50% of patients after surgery, it improves slowly over several weeks in most patients without symptoms. In some patients, however, hypoxia can progress to dyspnea. Impaired gas exchange can be induced by the reduction in muscle tension caused by anesthesia, decreases in respiratory capacity, tidal volume, functional residual capacity and diaphragm dysfunction caused by surgery elevated abdominal pressure and pain. In particular, post-operative fluid therapy, transfusion, inflammatory reaction, and sepsis are factors that aggravate respiratory failure. Positive pressure ventilation may be the mainstay of ARF treatment. A randomized prospective study of patients who underwent thoraco-abdominal surgery for aortic aneurysm and were treated with CPAP or oxygen showed that CPAP decreased the rate of intubation, increased the level of oxygen saturation and shortened hospital stay [9]. Other studies have also shown that positive pressure respiration after surgery is effective prophylactically in assisting pulmonary function lost during surgery, and therapeutically in treating patients with post-operative respiratory failure, with NIV being successful in most patients [15-17].
During the initial period of NIV use in our institution, the mask did not adhere closely to the face, resulting in air leakage, skin compression by the mask and receipt of excessive pressure. Thus, the skin became necrotized and patients were quite uncomfortable, contributing to the NIV failure. Due to improvements of mask materials, especially the use of helmet masks that are not in direct contact with the patient's face, patient discomfort and complications have been reduced, increasing patient compliance with NIV [10]. Moreover, nurses and physicians have gained more experience with NIV including both manipulation methods and patient education. Thus, of our 15 patients treated with NIV therapy, only 5 (33.3%) were converted to invasive ventilation during treatment, whereas the remaining 10 (66.7%) were treated successfully with NIV without endotracheal intubation. Others have also reported that 30 to 50% of patients are converted to invasive ventilation requiring intubation. Factors predicting failure of NIV include pneumonia, disease severity, older age and lack of improvement 1 hour after application of NIV [18,19]. Among our 5 patients who failed NIV and were converted to invasive ventilation, compliance with NIV was determined within 3 days of its application and mean period of invasive ventilation after conversion was 27 days, longer than in other patients who received mechanical ventilation. Our findings suggest that the selection of appropriate indications for NIV is very important, and that the delay of appropriate treatments could prolong the treatment period.
In NIV, positive pressure ventilation is applied with the cooperation of patients, thus preventing hypotension caused by the use of sedatives, shortening treatment periods, reducing complications associated with intubation and reducing the incidence of pneumonia. Nonetheless, NIV may be associated with complications such as gastric distention and pulmonary aspiration, and NIV may impede the ability of patients to cough and expectorate particularly in patients later converted to invasive ventilation.
Of ARF patients weaned from mechanical ventilation as planned, 6 to 23% require re-intubation with 48 to 72 hours [20-22]. This is due primarily to obstruction of the upper airways, insufficient coughing or expectoration, deterioration of consciousness, or myocardial failure. Although re-intubation reflects disease severity, intubation itself is an independent factor associated with increases in rates of pneumonia and death and duration of hospitalization [21,23]. Therefore, prevention of re-intubation is very important in the treatment of critically ill patients. In patients who developed respiratory failure after weaning, NIV was unable to lower mortality or re-intubation rate [23]. However high risk patients who received NIV soon after extubation, without re-intubation, could be successfully weaned from mechanical ventilation with a reduced mortality rate within the intensive care unit [24]. Similarly, all 3 of our patients who developed ARF after extubation were treated successfully with NIV without re-intubation.
Due to the retrospective design of our study, we could not control for factors such as the disease causing ARF at the time of therapeutic intervention, disease severity and clinical symptoms. This study, however, is to compare NIV with invasive ventilation in surgical patients in Korea. Prospective analysis of the prophylactic and therapeutic effects of NIV are therefore required.
In conclusion, we found that NIV may be successful in surgical patients who develop ARF, particularly in selected patients with post-operative atelectasis. The severity of ARF or hypoxia at the time of intervention was not associated with the effects of NIV. Moreover, NIV can successfully prevent re-intubation in patients who develop respiratory failure after extubation.
Figures and Tables
References
1. Warner DO. Preventing postoperative pulmonary complications: the role of the anesthesiologist. Anesthesiology. 2000. 92:1467–1472.
2. Vassilakopoulos T, Mastora Z, Katsaounou P, Doukas G, Klimopoulos S, Roussos C, et al. Contribution of pain to inspiratory muscle dysfunction after upper abdominal surgery: a randomized controlled trial. Am J Respir Crit Care Med. 2000. 161(4 Pt 1):1372–1375.
3. Lawrence VA, Dhanda R, Hilsenbeck SG, Page CP. Risk of pulmonary complications after elective abdominal surgery. Chest. 1996. 110:744–750.
4. Jaber S, Chanques G, Jung B. Postoperative Non-invasive ventilation. Anesthesiology. 2010. 112:453–461.
5. Mehta S, Hill NS. Non-invasive ventilation. Am J Respir Crit Care Med. 2001. 163:540–577.
6. Pennock BE, Kaplan PD, Carlin BW, Sabangan JS, Magovern JA. Pressure support ventilation with a simplified ventilatory support system administered with a nasal mask in patients with respiratory failure. Chest. 1991. 100:1371–1376.
7. Ambrosino N, Foglio K, Rubini F, Clini E, Nava S, Vitacca M. Non-invasive mechanical ventilation in acute respiratory failure due to chronic obstructive pulmonary disease: correlates for success. Thorax. 1995. 50:755–757.
8. Räsänen J, Heikkilä J, Downs J, Nikki P, Väisänen I, Viitanen A. Continuous positive airway pressure by face mask in acute cardiogenic pulmonary edema. Am J Cardiol. 1985. 55:296–300.
9. Kindgen-Milles D, Müller E, Buhl R, Böhner H, Ritter D, Sandmann W, et al. Nasal-continuous positive airway pressure reduces pulmonary morbidity and length of hospital stay following thoracoabdominal aortic surgery. Chest. 2005. 128:821–828.
10. Conti G, Cavaliere F, Costa R, Craba A, Catarci S, Festa V, et al. Non-invasive positive-pressure ventilation with different interfaces in patients with respiratory failure after abdominal surgery: a matched-control study. Respir Care. 2007. 52:1463–1471.
11. Jaber S, Delay JM, Chanques G, Sebbane M, Jacquet E, Souche B, et al. Outcomes of patients with acute respiratory failure after abdominal surgery treated with Non-invasive positive pressure ventilation. Chest. 2005. 128:2688–2695.
12. Antonelli M, Conti G, Bufi M, Costa MG, Lappa A, Rocco M, et al. Non-invasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA. 2000. 283:235–241.
13. Hilbert G, Gruson D, Vargas F, Valentino R, Gbikpi-Benissan G, Dupon M, et al. Non-invasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001. 344:481–487.
14. Feltracco P, Serra E, Barbieri S, Milevoj M, Furnari M, Rizzi S, et al. Non-invasive ventilation in postoperative care of lung transplant recipients. Transplant Proc. 2009. 41:1339–1344.
15. Squadrone V, Coha M, Cerutti E, Schellino MM, Biolino P, Occella P, et al. Continuous positive airway pressure for treatment of postoperative hypoxemia: a randomized controlled trial. JAMA. 2005. 293:589–595.
16. Antonelli M, Conti G, Rocco M, Bufi M, De Blasi RA, Vivino G, et al. A comparison of Non-invasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med. 1998. 339:429–435.
17. Antonelli M, Conti G, Esquinas A, Montini L, Maggiore SM, Bello G, et al. A multiple-center survey on the use in clinical practice of Non-invasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007. 35:18–25.
18. Wallet F, Schoeffler M, Reynaud M, Duperret S, Workineh S, Viale JP. Factors associated with Non-invasive ventilation failure in postoperative acute respiratory insufficiency: an observational study. Eur J Anaesthesiol. 2010. 27:270–274.
19. Antonelli M, Conti G, Moro ML, Esquinas A, Gonzalez-Diaz G, Confalonieri M, et al. Predictors of failure of Non-invasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi-center study. Intensive Care Med. 2001. 27:1718–1728.
20. Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest. 1997. 112:186–192.
21. Esteban A, Alía I, Tobin MJ, Gil A, Gordo F, Vallverdú I, et al. Spanish Lung Failure Collaborative Group. Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Am J Respir Crit Care Med. 1999. 159:512–518.
22. Torres A, Gatell JM, Aznar E, el-Ebiary M, Puig de la Bellacasa J, González J, et al. Re-intubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation. Am J Respir Crit Care Med. 1995. 152:137–141.
23. Esteban A, Frutos-Vivar F, Ferguson ND, Arabi Y, Apezteguía C, González M, et al. Non-invasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004. 350:2452–2460.
24. Ferrer M, Valencia M, Nicolas JM, Bernadich O, Badia JR, Torres A. Early Non-invasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med. 2006. 173:164–170.




 ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download