Abstract
Purpose
Laparoscopic liver resection (LLR) is now widely accepted and is being increasingly performed. The present study describes our experience with LLR at a single center over an eight-year period.
Methods
This retrospective study enrolled 100 patients between October 2002 and February 2010. Forty-six benign lesions and 54 malignant lesions were included. The LLR performed included 58 pure laparoscopy procedures, 18 hand-assisted laparoscopy procedures and 24 hybrid technique procedures.
Results
The mean age of the patients was 57 years; among these patients, 31 were over 65 years of age. The mean operation time was 220 minutes. The overall morbidity was 11% and the mortality was zero. Among the 20 patients with simple hepatic cysts, 50% unexpectedly recurred. Among the 41 patients with hepatocellular carcinoma, 21 patients (51%) underwent preoperative radiofrequency ablation therapy or transarterial chemoembolization. During parenchymal-transection, 11 received blood transfusion. The width of the resection margins was under 0.5 cm in 11 cases (27%); 0.5 to 1 cm in 22 cases (54%) and over 1 cm in eight cases (12%). There was no port site seeding, but argon beam coagulation-induced tumor dissemination was observed in two cases. The overall two-year survival rate was 75%.
With the recent advances of the techniques and devices used for laparoscopic liver resection (LLR), the number of surgical departments that perform these procedures has gradually increased [1,2]. Our center initially started performing LLR for benign cysts, deroofing and peripheral tumor resection via non-anatomic wedge resection procedures. With the aid of several new techniques, hand-assisted laparoscopy (HALS) and the laparoscopic assisted "hybrid" techniques, resection of the posterior superior segment and major anatomical resections have recently become feasible. Each surgical center may differ in their patient selection and the protocol used for LLR procedures [2]. During the early era of laparoscopic cholecystectomy, the frequency of bile duct injuries was increased two fold with the laparoscopic approach. However, the complications have gradually decreased, and the interest has in these procedures has increased [3]. Bleeding was the most serious intraoperative complication, in addition to the development of a gas embolism. Tumor seeding and perioperative recurrence have also been controversial, when LLR was performed in patients with a malignancy. However, these concerns have been addressed, and the indications for LLR have expanded from benign to malignant lesions; the role of the LLR continues to increase among surgical procedures [4,5].
From October 2002 to February 2010, 100 patients underwent LLR for various disease at the Department of Surgery, Dong-A Medical Center, Busan, Korea, and we retrospectively evaluated these patients' data. Forty six benign lesions and 54 malignant lesions were included. The LLR performed included 58 pure laparoscopy procedures, 18 HALS procedures and 24 hybrid technique procedures.
Preoperatively, good quality imaging of the liver with ultrasound and/or computed tomography scan, magnetic resonance imaging and/or positron emission tomography scan is needed for the cases with possible intrahepatic metastasis [6]. In brief, the LLR procedure at our institution was as follows: the resection was performed with the patient in the lithotomy position, with the surgeon standing between the patients' legs and the scopist was always sitting to avoid interference with the surgical instruments. The liver resection was defined according to Couinaud's classification. Hepatectomy was considered anatomic when at least one segment was entirely removed, and all the other resections were defined as non-anatomic [1]. Pure laparoscopic procedures were used for the hepatectomies for the lesions located on the surface or inferolateral segments (II, III, IVa, V, and VI). A hand-assisted laparoscopic hepatectomy or a hybrid procedure was used for resection of the lesions located at the posterosuperior segments of the liver (VII, VIII, and IVb), for large tumors more than 5 cm and for major hepatic resections (three segments or more). During the liver parenchymal resection, to decrease bleeding, the central venous pressure was maintained at 2 to 5 cm H2O whenever possible. In addition, low intra-abdominal pressure was strictly maintained at 8 to 10 mmHg during the procedure to prevent a gas embolism. After demarcation of the transection line on the surface of the liver, the superficial liver parenchyma was divided using a harmonic scalpel (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA) and the deeper portion was divided using a laparoscopic cavitron ultrasonic surgical aspirator (Valleylab, Boulder, CO, USA). Compression using small gauze was used to control bleeding during the LLR. An endoscopic gastrointestinal anastomosis stapler may be helpful for dealing with injury to the lateral wall of the major vessels. Mobilization and lifting of the liver upward can decrease the inflow to the liver, as another option [2,7]. For the cirrhotic liver, to prevent bleeding, precoagulation with high voltage (70 to 100 V) monopolar coagulation was performed along the transection line; the "kellyclasia" technique was useful in some cases. To preserve the functional liver parenchyma, and to not compromise the oncological integrity of the cancer, various negative resection margins were attempted, according to the status of the liver parenchyma. If the remaining liver parenchyma was relatively healthy, then the distance from the tumor to the resection margins was more than 1 cm; however, in severe, cirrhotic livers it was between 0.5 to 1 cm. In the patients with a previous history of radiofrequency ablation (RFA) or transarterial chemoembolization (TACE), 0.5 cm was attempted as a safe resection margin.
A total of 100 LLR were performed from October 2002 to February 2010. Of the 100 patients with various diseases and who underwent LLR were 42 men and 58 were women with a mean age of 57 (range, 23 to 87 years). The mean operation time was 222 minutes (range, 30 to 665 minutes). The hospital stays of the 100 patients ranged from 3 to 42 days (mean, 12.4 days) (Table 1).
The liver lesions among the patients are listed in Table 1. The number of LLR procedures has progressively increased since 2007 and the proportion of malignant lesions, and especially hepatocellular carcinoma (HCC), has increased dramatically (Fig. 1). With regards to the tumor location, the tumors of 25 patients in the first half of the study and tumors of 44 patients in the second half of the study were in the peripheral portion of the anterolateral segments of the liver (Segments II, III, V and VI and the inferior part of IV). Whereas, the tumors of 8 patients in the first half of the study and 18 patients in the second half of the study were in the posterior or superior part of the liver (Segments I, VII and VIII and the superior part of IV). There were multiple tumors in 5 patients. The types of LLR performed are summarized in Table 2. There were 12 left lateral sectionectomies, 9 left hemihepatectomies, 6 right hemihepatectomies, 2 posterior sectionectomies, and 2 central bisegmentectomy. As for the type of LLR, there were 58 pure laparoscopies, 18 HALS and 24 hybrid techniques.
One out of the 100 procedures was converted to open surgery due to uncontrollable bleeding. Eight patients had a history of previous abdominal surgery and one repeat LLR was performed. The resected lesions were located in all the liver segments, except for segment I. Eighty six were single lesions and 14 were multiple lesions. There was no patient death. The overall morbidity was 11%, including eight grade I complications (ascites, pulmonary complication, liver dysfunction) and three grade 3 complications (biloma that required percutaneous drainage). The majority of the LLR procedures in the benign cases were performed for simple hepatic cysts (20 cases); LLR is currently the preferred surgical approach for giant hepatic cysts and polycystic liver disease.
After laparoscopic deroofing, cyst recurrence (defined as more than the half diameter of the cyst postoperatively) occurred in 50% of the cases, and this was unexpected (Fig. 2). The remainder of the benign lesions included 12 intrahepatic stones, 5 hemangiomas and 2 lesions with focal nodular hyperplasia, and 2 procedures were performed for biopsies. A total of 54 patients underwent LLR procedures for malignant pathology, including 41 for HCC, 4 for cholangiocarcinoma, 5 for metastatic colorectal adenocarcinoma and 4 for gallbladder carcinoma. Among the 41 patients with HCC, 25 were hepatitis B positive and the diameter of the lesion was under 2 cm in 13 cases, between 2 and 5 cm in 23 cases and over 5 cm in 5 cases. Twenty one (43%) of these patients had preoperative procedures: 10 had RFA, 9 underwent TACE, 1 received systemic chemotherapy and 1 had an open hepatectomy. Thirteen (28%) of the patients with HCC underwent the following procedures postoperatively: 2 received RFA, 4 received TACE, 6 received systemic chemotherapy and 1 underwent alcohol injections. Thirty four patients had single nodules and the tumor location was in the left lateral section in 11 cases, the inferior segments in 23 cases and the posterosuperior segment in 14 cases. LLR was performed purely laparoscopically in 23 cases, 8 underwent HALS and 10 underwent hybrid procedures (Table 2).
For the analysis, our experience was divided into two halves of the study. The first 17 cases were performed before February 2009, and the second half included 24 cases as well as the strict maintenance of the central venous pressure at 2 to 5 cmH2O to decrease bleeding during the liver parenchymal transection. In the first half, 5 patients required a transfusion, and the mean amount of blood transfusion amount was 346 mL. In the second half, 6 patients required a transfusion, and the mean amount of blood transfusion was 287 mL; the difference was not statistically significant (P = 0.45). For the 41 patients with malignant pathology, the width of the specimen margins was under 0.5 cm in 11 cases (27%), 0.5 to 1 cm in 22 cases (54%) and over 1 cm in 8 cases (19.5%). Microscopic portal vein thrombosis was identified in 8 cases (19.5%). The width of the resection margin was compared macroscopically and microscopically among the fresh specimens and then after fixation in formaldehyde; there was a 30% reduction of the width of the resection margin among the specimens that were microscopically evaluated (data not shown). No port site metastasis was noted during the follow-up period. The tumor recurrence that occurred in two cases was related to the high pressure argon beam coagulator used for hemostasis; the increased intratumor pressure possibly promoted tumor cell dissemination through the adjacent venous system, and this was characterized by aggressive spreading and frequent retroperitoneal organ metastasis within a short interval (Fig. 3). During the follow up period, 10 cases (25%) had recurrent disease and 6 patients died. The overall 2-year survival rate was 75%. The follow-up ranged from 0.3 to 71 months (mean, 16 months).
The reproducibility and feasibility of LLR has improved in terms of the operative time, the conversion rate, the blood loss, the hospital stay and the morbidity and mortality. The frequency of LLR has recently significantly increased [1-7]. Recent reports have suggested that LLR is particularly useful for elderly patients with regard to an earlier recovery and reduced morbidity [2,8]. In our current series, 31% of the cases were more than 65 years of age. However, the increased use of LLR might be too rapid and overly optimistic [8].
The technique for liver parenchymal transection has advanced with the improvement in the magnification and depth of vision of the vascular structures [5]. A pure laparoscopic procedure has generally been used for non-anatomic resection for tumors located on the surface of the inferolateral segments (segments II, III, IVa, V and VI), and HALS procedures for the non-anatomic resection of tumors located in the posterosuperior segments (segments VII, VIII and IVb). Hybrid procedures have the benefits of both open and laparoscopic procedures and they may increase the indications, as well as the safety, of the LLR [2,9,10].
Intraoperative complications such as hemorrhage or the inability to make progress might make pure laparoscopy procedures less desirable than the HALS procedure or hybrid procedures. In this current series, the unplanned use of HALS or the hybrid technique during a pure laparoscopic procedure had recently increased. The surgical indications for benign cystic lesions should be very limited and they include patients that are symptomatic (mass effect, abdominal pain, vegetative symptoms and dyspnea) and those who have the potential for bleeding, infection or malignant transformation [3,10]. The majority of our cases with surgical indications had giant hepatic cysts; the remaining cases included hepatolithiasis, hemangiomas, focal nodular hyperplasia and cystadenomas. Treatment for the patients with giant hepatic cysts was reserved for those with a mass effects, bleeding and infection and to rule out a malignancy. During the deroofing process, special care should be taken not to resect the hepatic parenchyma, given that a transected bile duct may lead to a postoperative bile leak. Giant hepatic cysts located in the right posterior lobe have a high tendency to recur because of the close contact between the liver and diaphragm, which interferes with adequate drainage of the deroofed cyst and this leads to the reaccumulation of its contents. Laparoscopic deroofing provided complete relief for both simple hepatic cysts and polycystic liver disease, and it has a reported recurrence rate of 2 to 5% [11,12]. However, in this series, among the simple cysts and the one pathologically proven cystadenoma, 50% recurred, and these patients are now being treated conservatively. Although the rate of recurrence was high, almost all the patients' symptoms were significantly improved. This data suggests that the frequency of simple hepatic cyst recurrence was unexpectedly high, so new and more effective treatment modalities are needed. For the low rates of cyst recurrence, we can perform careful electrocoagulation of the remaining lining of the cyst wall after deroofing, cystojejunostomy, alcoholization (alcohol sclerosis) and radiofrequency ablation [12]. In cases with a strong suspicion of a biliary communication or adenocarcinoma, the surgeon should proceed with the resection, with careful fluid analysis of the cyst for the carbohydrate antigen 19-9 and carcinoembryonic antigen levels and performing a cyst wall biopsy for the histology [13].
The use of LLR has recently expanded to malignant lesions, and mainly colorectal metastasis and HCC, as well as for major hepatectomies [14]. For colorectal metastasis, a normal underlying liver allows for extensive resection without postoperative hepatic failure and preservation of a sufficient safety margin. However, there is concern that small metastases can be missed during the LLR; therefore, intraoperative laparoscopic ultrasonography is essential [15]. The laparoscopic ultrasound supports achieving an adequate resection margin. A positive margin has been shown to predict poor disease-free survival, yet the width of a negative margin has not been correlated with recurrence or survival. The majority of the LLR procedures that have been done for malignancies have been performed in patients with HCC. LLR for HCC in properly selected cirrhotic patients results in fewer early postoperative complications and a shorter hospital stay compared to the traditional open hepatectomy. The specific benefits from LLR in cirrhotic patients include 1) preservation of the collateral veins and this results in a reduced frequency of portal hypertension, 2) a decrease in the mobilization and manipulation of the liver, 3) minimal insensible fluid loss, and 4) a reduced risk of postoperative accumulation of ascites [9,16,17].
RFA has recently gained favor as a minimally invasive treatment that might replace LLR for treating patients with HCC [16]. In this series, the rate of performing pre and post operative ablative therapy gradually increased. However, RFA is not ideal for superficially located HCC because of the increased risk of bleeding, tumor seeding and thermal injury to adjacent organs [2,17-19].
The major problem encountered during the LLR is the difficulty with controlling bleeding and providing an adequate oncologic margin. Bleeding is the most serious intraoperative complication. Maintaining the central venous pressure below 2 to 5 cm H2O by a cautious anesthesiology team is essential [20,21]. Some studies have reported that low central venous pressure (CVP) during liver resection could significantly cut down the intra-operative blood loss, decrease the incidence of post-operative complications and shorten the hospital stay [22,23]. With the patient under hepatic hilum occlusion, the blood loss during liver resection is mainly derived from the hepatic vein and short hepatic vein. The hepatic sinusoidal pressure is directly related to the CVP. With lowering the pressure in the inferior vena cava, the hepatic venous pressure and then the hepatic sinusoidal pressure will decline. The blood loss during liver resection is proportional to the pressure gradient of the vascular walls and the diameter of the injured vessels [24,25]. In this series, a central venous pressure above 8 cm H2O resulted in a higher rate of blood transfusions during the first half of the study; during the second half of the study, the central venous pressure was lowered to 2 to 5 cm H2O. Although the transfusion rate was lower during the second half of the study, the difference was not statistically significant. Strict maintenance of a low intra-abdominal pressure below 8 to 10 mmHg is important to prevent a CO2 gas embolism. Particularly in cirrhotic patients, to prevent bleeding, the resection line is diathermically precoagulated before the parenchymal transection. In the cirrhotic liver, a limited resection is an important approach to avoid postoperative liver failure; functional liver parenchyma should not be sacrificed to obtain a wide margin [21]. A positive histological margin was associated with a higher frequency of postoperative recurrence, but the width of the resection margin was not associated with postoperative recurrence after hepatectomy in patients with HCC. Most of the cases of intrahepatic recurrence in our study were thought to be due to venous dissemination, which a wide resection margin could not prevent [16]. A positive surgical margin was defined as the presence of exposed tumor along the line of transection or the presence of tumor cells at the line of transection, as detected by histological examination. However, an accurate assessment of the surgical margin during LLR can be difficult [26]. One reason for this is that the ultrasonic dissector used to aspirate a portion of the liver parenchyma causes the liver parenchyma to crack, resulting in the potential for overestimating the true rate of positive margins [21,26]. In this series, the formaldehyde fixed specimens and the fresh specimens were compared with regard to the microscopically evaluated resection margins and the macroscopically measured resection margins; there was on average a 30% underestimation of the width of the resection margin after fixation. Controversy exists as to the adequacy of tumor excision and the resection margins; in a previous large series the median resection margins of 5 and 10 mm were reported for colorectal metastasis and HCC, respectively [10,20,27].
In this current series, the cases that underwent preoperative ablation therapy (RFA or TACE) had encapsulated cirrhosis and a 5 to 10 mm safety margin, or colorectal metastasis with a normal liver and more than a 10 mm safety margin. When LLR is performed in patients with HCC or metastatic colorectal cancer, there must be no compromise with regard to the oncologic integrity during surgery; achieving negative margins should always be attempted [28]. The largest series published to date, which was without the long-term follow-up for cancer recurrence, reported no episodes of tumor seeding and no port site recurrence when compared with that of the open resection group.
CO2 pneumoperitoneum is considered to be much safer than air embolism because of the greater solubility of CO2 compared to that of nitrogen. The occurrence of a gas embolism has been related to argon beam coagulation, which increases the endo-abdominal pressure and causes an increased risk for a gas embolism. Thus, it is important to select a low flow setting and achieve adequate venting through the laparoscopic ports to maintain a safe pressure between 8 and 12 mmHg [8]. Using an argon beam coagulator for hemostasis, and especially for bleeding from a significant vessel, raises additional concern about the possible high intralesional pressure that will facilitate tumor dissemination; this might occur through the venous system and so this, account for early intrahepatic or extrahepatic recurrence. In addition, RFA often causes vaporization of intracellular water and the formation of microbubbles within the ablation zone; the resulting high intralesional pressure might well facilitate tumor dissemination through the venous system [20,29-32]. In our current series, 2 cases of retroperitoneal and adrenal gland seeding, which were possibly due to using an argon beam coagulator for hemostasis, were characterized by rapid, aggressive dissemination through the venous system. After this experience, argon gas embolization was abandoned for hemostasis during parenchymal transection.
In conclusion, LLR can not totally replace open hepatectomy procedures, yet it is a useful treatment option for liver surgery, and especially for some groups of patients such as those with cirrhotic liver disease and the elderly. HALS and hybrid techniques have recently expanded LLR's applications. Recurrence was unexpectedly high for benign simple cysts and so more effective treatment modalities are needed. For the malignant cases, lowering the central venous pressure was found to be essential in order to reduce bleeding. Measuring the resection margin before and after fixation can affect the accuracy of this measurement. In addition, the determination of an adequate resection margin can change according to the status of the liver parenchyma and a history of previous ablation therapy. An argon beam coagulator must be cautiously used with maintaining an adequate tumor margin to prevent cancer dissemination. The findings of this study show that the gradual expansion of the indications for LLR should proceed only when there is data supporting that its safety and efficacy for treating patients is equivalent to that of open liver resection.
Figures and Tables
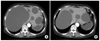
Fig. 2
Recurrent hepatic cysts. (A) Preoperative abdominal computed tomography shows multiple hepatic cysts, both lobes of the liver. (B) Deroofing 7 months after, re-expansion of a giant hepatic cyst in right lobe.
References
1. Vibert E, Perniceni T, Levard H, Denet C, Shahri NK, Gayet B. Laparoscopic liver resection. Br J Surg. 2006. 93:67–72.
2. Otsuka Y, Tsuchiya M, Maeda T, Katagiri T, Isii J, Tamura A, et al. Laparoscopic hepatectomy for liver tumors: proposals for standardization. J Hepatobiliary Pancreat Surg. 2009. 16:720–725.
3. Buell JF, Thomas MT, Rudich S, Marvin M, Nagubandi R, Ravindra KV, et al. Experience with more than 500 minimally invasive hepatic procedures. Ann Surg. 2008. 248:475–486.
4. Koffron AJ, Auffenberg G, Kung R, Abecassis M. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg. 2007. 246:385–392.
5. Vigano L, Laurent A, Tayar C, Tomatis M, Ponti A, Cherqui D. The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg. 2009. 250:772–782.
6. Bryant R, Laurent A, Tayar C, Cherqui D. Laparoscopic liver resection-understanding its role in current practice: the Henri Mondor Hospital experience. Ann Surg. 2009. 250:103–111.
7. Han HS, Cho JY, Yoon YS. Techniques for performing laparoscopic liver resection in various hepatic locations. J Hepatobiliary Pancreat Surg. 2009. 16:427–432.
8. Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, Dagher I, et al. The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg. 2009. 250:825–830.
9. Kaneko H, Tsuchiya M, Otsuka Y, Yajima S, Minagawa T, Watanabe M, et al. Laparoscopic hepatectomy for hepatocellular carcinoma in cirrhotic patients. J Hepatobiliary Pancreat Surg. 2009. 16:433–438.
10. Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009. 250:831–841.
11. Koffron A, Geller D, Gamblin TC, Abecassis M. Laparoscopic liver surgery: shifting the management of liver tumors. Hepatology. 2006. 44:1694–1700.
12. Mazza OM, Fernandez DL, Pekolj J, Pfaffen G, Sanchez Clariá R, Molmenti EP, et al. Management of nonparasitic hepatic cysts. J Am Coll Surg. 2009. 209:733–739.
13. Koffron A, Rao S, Ferrario M, Abecassis M. Intrahepatic biliary cystadenoma: role of cyst fluid analysis and surgical management in the laparoscopic era. Surgery. 2004. 136:926–936.
14. Dagher I, O'Rourke N, Geller DA, Cherqui D, Belli G, Gamblin TC, et al. Laparoscopic major hepatectomy: an evolution in standard of care. Ann Surg. 2009. 250:856–860.
15. Nguyen KT, Laurent A, Dagher I, Geller DA, Steel J, Thomas MT, et al. Minimally invasive liver resection for metastatic colorectal cancer: a multi-institutional, international report of safety, feasibility, and early outcomes. Ann Surg. 2009. 250:842–848.
16. Poon RT, Fan ST, Ng IO, Wong J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal. Ann Surg. 2000. 231:544–551.
17. Cherqui D, Laurent A, Tayar C, Chang S, Van Nhieu JT, Loriau J, et al. Laparoscopic liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: midterm results and perspectives. Ann Surg. 2006. 243:499–506.
18. Masuda T, Beppu T, Ishiko T, Horino K, Baba Y, Mizumoto T, et al. Intrahepatic dissemination of hepatocellular carcinoma after local ablation therapy. J Hepatobiliary Pancreat Surg. 2008. 15:589–595.
19. Ng KK, Poon RT, Lo CM, Yuen J, Tso WK, Fan ST. Analysis of recurrence pattern and its influence on survival outcome after radiofrequency ablation of hepatocellular carcinoma. J Gastrointest Surg. 2008. 12:183–191.
20. Spencer L, Metcalfe MS, Strickland AD, Elsey EJ, Robertson GS, Lloyd DM. Lessons from laparoscopic liver surgery: a nine-year case series. HPB Surg. 2008. 2008:458137.
21. Kazaryan AM, Pavlik Marangos I, Rosseland AR, Røsok BI, Mala T, Villanger O, et al. Laparoscopic liver resection for malignant and benign lesions: ten-year Norwegian single-center experience. Arch Surg. 2010. 145:34–40.
22. Johnson M, Mannar R, Wu AV. Correlation between blood loss and inferior vena caval pressure during liver resection. Br J Surg. 1998. 85:188–190.
23. Smyrniotis V, Kostopanagiotou G, Theodoraki K, Tsantoulas D, Contis JC. The role of central venous pressure and type of vascular control in blood loss during major liver resections. Am J Surg. 2004. 187:398–402.
24. Wang WD, Liang LJ, Huang XQ, Yin XY. Low central venous pressure reduces blood loss in hepatectomy. World J Gastroenterol. 2006. 12:935–939.
25. Uchiyama K, Ueno M, Ozawa S, Hayami S, Kawai M, Tani M, et al. Half clamping of the infrahepatic inferior vena cava reduces bleeding during a hepatectomy by decreasing the central venous pressure. Langenbecks Arch Surg. 2009. 394:243–247.
26. Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005. 241:715–722.
27. Tralhão JG, Kayal S, Dagher I, Sanhueza M, Vons C, Franco D. Resection of hepatocellular carcinoma: the effect of surgical margin and blood transfusion on long-term survival. Analysis of 209 consecutive patients. Hepatogastroenterology. 2007. 54:1200–1206.
28. Viganò L, Tayar C, Laurent A, Cherqui D. Laparoscopic liver resection: a systematic review. J Hepatobiliary Pancreat Surg. 2009. 16:410–421.
29. Ohno T, Kawano K, Yokoyama H, Tahara K, Sasaki A, Aramaki M, et al. Microwave coagulation therapy accelerates growth of cancer in rat liver. J Hepatol. 2002. 36:774–779.
30. Ikegami T, Shimada M, Imura S, Nakamura T, Kawahito S, Morine Y, et al. Argon gas embolism in the application of laparoscopic microwave coagulation therapy. J Hepatobiliary Pancreat Surg. 2009. 16:394–398.
31. Min SK, Kim JH, Lee SY. Carbon dioxide and argon gas embolism during laparoscopic hepatic resection. Acta Anaesthesiol Scand. 2007. 51:949–953.
32. Min BS, Lee KY, Park JK, Kim NK, Lee JT, Min JS. Radiofrequency ablation of hepatic metastasis from colorectal cancer: early experience. J Korean Surg Soc. 2002. 62:145–149.




 Citation
Citation Print
Print





 XML Download
XML Download