Abstract
Purpose
The aim of this study was to assess the role of pre-operative chest computed tomography (CT) compared with abdominopelvic CT (AP-CT) and chest radiography (CXR) for detecting pulmonary metastasis in patients with primary colorectal cancer (CRC).
Methods
We retrospectively analyzed the data of 619 patients with primary CRC who simultaneously received a preoperative chest CT (chest CT group), AP-CT with hilar extension, and CXR (CXR group).
Results
In the chest CT group, there were 297 (48.0%) normal, 198 (32%) benign, 96 (15.5%) indeterminate, 26 (4.2%) metastasis, and two lung cancers. Eighteen patients (2.9%) in the CXR group who had no pulmonary metastasis were diagnosed with pulmonary metastasis on a chest CT. The sensitivity and accuracy were 83.9% and 99.0% in the chest CT group, respectively, and 29.0% and 91.5% in the CXR group, respectively (P < 0.0001 and P = 0.0003).
The lung is the second most common distant metastatic site for primary colorectal cancer (CRC), and about 10% of all patients with CRC develop pulmonary metastasis [1]. However, fewer than 10% of the patients are candidates for pulmonary metastasectomy [2]. Since Blalock [3] first reported the successful surgical resection of a pulmonary metastasis from colorectal cancer in 1994, and Thomford et al. reported the principles of pulmonary metastasis surgical resection, the procedure has been gradually accepted as a treatment for pulmonary metastasis and many physicians have reported the value of surgical resection for pulmonary metastases [4-6].
Traditionally, pulmonary staging for CRC has been conducted by chest radiography (CXR), but CXR has low sensitivity and specificity. According to the National Comprehensive Cancer Network (NCCN) guidelines v. 1. 2007, chest computed tomography (CT) is recommended instead of CXR for the pre-operative workup of CRC patients [7,8]. Although a chest CT scan is more sensitive than CXR for the detection of pulmonary metastases, not all lesions observed on a chest CT of patients with CRC will be metastases [9]. Another study reported that CXR was no more useful for detecting early pulmonary metastases after curative colorectal surgery for stage III CRC than chest CT or abdominal CT [10].
We investigated the role of pre-operative chest CT compared with abdominopelvic CT (AP-CT) with hilar extension and CXR, which was the previous standard method for detecting pulmonary metastasis in patients with primary CRC.
Between January and June 2008, 767 consecutive patients who were diagnosed with CRC at Samsung Medical Center, Seoul, Korea were simultaneously checked preoperatively with CXR, AP-CT with hilar extension (CXR group), and chest CT (chest CT group). The upper limit of hilar extension was set at 3 cm above the highest point of the diaphragm. Chest CTs and CXRs were reviewed by a radiologist in the chest division, and the AP-CTs were reviewed by radiologists in the abdominal division. According to the radiologic findings, we divided the patients into four categories: normal, benign, metastatic, and indeterminate [11,12]. A pulmonary metastasis was defined as a nodule that had spiculation, infiltration, or a monogonal shape and was ≥5 mm in size. An indeterminate nodule was defined as a nodule that had no calcification and was <5 mm in size (Fig. 1). Patients who had benign or indeterminate nodules had a follow-up chest CT within 3 months, and all patients were routinely checked 6 months later. Exclusion criteria were: 1) unknown results of the preoperative chest CT; 2) less than a 6-month follow-up duration.
A total of 619 patients were included in this study, and we retrospectively analyzed the collected data. Three-hundred seventy-three of the patients were male (60.3%), and 246 were female (39.7%), with a mean age of 59.4 ± 11.6 years (range, 23 to 88 years).
The McNemar test and Bennett's method were used to compare the sensitivity, specificity, predictive value, and accuracy between the chest CT group and CXR group. A P-value < 0.05 was considered statistically significant.
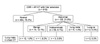
Three-hundred twenty-two (52.0%) out of 619 patients showed radiologic abnormality on their chest CT. Among these patients, the lesions of 198 patients (32.0%) were diagnosed as benign nodules, 96 patients (15.5%) as indeterminate nodules, 26 patients (4.2%) as pulmonary metastases, and two patients (0.3%) as lung cancer on chest CTs (Fig. 2). In the CXR group, 135 patients (21.8%) showed radiologic abnormality and, among them, the lesions of 75 patients (12.1%) were diagnosed as benign nodules, the lesions of 50 patients (8.1%) were diagnosed as indeterminate nodules, and the lesions of 8 patients (1.3%) were diagnosed as pulmonary metastases; 2 patients (0.3%) were diagnosed with lung cancer (Fig. 3). Twelve patients (1.9%) were diagnosed with no active pulmonary lesion, one patient (0.2%) with benign nodules, and three patients (0.5%) with indeterminate nodules; two patients (0.3%) who were diagnosed with lung cancer in the CXR group were diagnosed with pulmonary metastases on chest CT (Table 1). Fourteen of the 26 patients who had pulmonary metastases on the pre-operative chest CT had their diagnosis confirmed by video-assisted thoracoscopic resection and 12 of the 26 were routinely followed-up with chemotherapy and/or radiotherapy (Fig. 4). Eighteen (2.9%) of the 26 patients with pulmonary metastases underwent a change in treatment strategy due to the preoperative detection of a pulmonary metastasis according to changes in the guidelines. Among these patients, 23 (3.7%) had isolated pulmonary metastases, 2 had simultaneous hepatic metastases, and 1 had peritoneal seeding.
In the chest CT group, four patients (0.6%) had incidental detection of combined lesions (two lung cancers, one thyroid cancer, and one thymoma) and they received curative surgery.
Five patients were diagnosed with pulmonary metastases on the follow-up chest CT. The preoperative chest CT of four of the five patients revealed benign nodules, and one of them was diagnosed with indeterminate nodules. The preoperative AP-CT showed that two of the five patients were normal, and three were diagnosed with benign nodules. All were normal on CXR.
Finally, 31 patients (5.0%) were diagnosed with pulmonary metastasis. Five of these patients (16.1%) had a distribution in the upper hilar area, 10 (32.3%) in the lower hilar area, and 16 (51.6%) in both areas (Table 2).
The sensitivity of the chest CT group was 83.9% and that of the CXR group was 29.0% (P < 0.0001). The negative predictive value for the detection of pulmonary metastases was 99.0% in the chest CT group and 91.4% in the CXR group (P = 0.0002). The accuracy was 99.0% in the chest CT group and 91.5% in the CXR group (P = 0.0003). There was no different value of the specificity and positive predictive value between the two groups.
The lung is one of the most frequent sites for CRC metastasis, which affects about 10% of all patients [1]. Isolated pulmonary metastasis occurs in only 1% of patients and is usually associated with another systemic metastasis such as the liver, brain, or bone [5,13]. Pulmonary metastases are usually unresectable due to multiple lesions but after a complete resection of pulmonary metastasis from CRC, there are significant improvements in survival and in long-term survivors; multiple series have reported 5-year survival rates of 30.5 to 55% [14-19]. Therefore, it is important to detect pulmonary metastases from primary CRC to identify treatment strategies for patients [20,21]. Ike et al. [22] reported that screening with chest CT and pulmonary resection improves the prognosis of pulmonary metastasis from CRC, whereas Schoemaker et al. [23] reported that there was no benefit of a yearly colonoscopy, liver CT, or CXR for prognosis after curative resection of CRC. Thus, early detection of pulmonary metastasis is important to improve the prognosis of resectable CRC with pulmonary metastases. Generally, the detection rate for plain chest radiography is lower than for CT, so AP-CT with a hilar extension could be helpful in detecting peripheral, especially lower, multiple metastatic nodules [24]. Currently, preoperative pulmonary staging for CRC is conducted by CXR, but CXR only identifies 1.8-12.0% of patients with resectable pulmonary metastases [25]. In this study, CXR detected only 9.7% (3/31) of pulmonary metastatic nodules. According to the NCCN guidelines v. 1. 2007, chest CT is recommended instead of CXR for preoperative CRC staging [7,8]. A chest CT scan is 51 to 73% more sensitive than CXR for the detection of pulmonary metastases [26]. The specificity of chest CT is also superior to that of CXR and has been reported to be about 74% accurate [27].
In this study we investigated the role of chest CT and compared it with AP-CT with a hilar extension and CXR, which were used previously for preoperative staging of primary CRC. The accuracy of the chest CT (99%) was better than AP-CT with hilar extension and CXR (91.5%) in detecting pulmonary metastases.
Pulmonary metastases were mainly hematogeneous spreading, multiple nodules, with peripheral and lower hilar locations, so they could be detected by AP-CT with a hilar extension [28,29]. Also in this study, 26 patients (83.9%) had metastatic lesions in the lower hilar or both lung fields. But the pulmonary metastases in the upper hilar area could not be detected with CXR and AP-CT with a hilar extension (in our study, about 16.1% were detected); thus, chest CT would be helpful for these patients. Although the AP-CT scan extended to the hilar, we did not find pulmonary metastases in 12 patients (38.7%). The reasons for the different detection rates between AP-CT and chest CT were: 1) AP-CT with a hilar extension can't detect metastases if the lesions are located in the upper hilar area; 2) the thickness of the slice is different between AP-CT and chest CT (5.0 mm vs. 2.5 mm); and 3) differences in the imaging method, i.e., AP-CT is conducted in a full expiration state, whereas chest CT is conducted in a full inspiration state, so if the metastasis is located in basal lung, the lung would be collapsed, and small-sized metastatic nodules wouldn't be detected by AP-CT.
In addition to chest CT's higher detection rate for pulmonary metastasis than the previous method, it provided more information as a preoperative work-up. For example, we were able to find other meaningful lesions such as lung cancer, thymoma, and thyroid cancer. A preoperative chest CT also could be used as a baseline study for follow-up of presumed benign and indeterminate lesions. In this study, five patients (four benign and one indeterminate) were diagnosed with pulmonary metastasis on the follow-up chest CT at 3 or 6 months due to a change in shape or increase in nodule size.
Pulmonary metastases were more common with rectal cancer than with colon cancer. In this study, 31 patients (5.0%) had pulmonary metastases and, among them, 12 (1.9%) had colon cancer and 19 (3.1%) had rectal cancer, although the differences were not significant (P = 0.081, Table 3).
Although the detection rate for pulmonary metastasis using a chest CT was higher, too many insignificant lesions were detected on pre-operative chest CT (52.0% vs. 21.8%), and they could be confusing when defining a proper treatment strategy. Although we didn't investigate the hazardous effect of radiation and cost effectiveness, the patients who had benign and indeterminate lesions on preoperative chest CT were checked by positron emission tomography or follow-up chest CT at short-term intervals.
In conclusion, although this study was limited by a short-term follow-up, our results suggest that the new guidelines, which include chest CT as a preoperative pulmonary work-up, are better than the previous guidelines, which included AP-CT with hilar extension and CXR for CRC. If benign or indeterminate lesions were detected on pre-operative chest CT, they should not be ignored and should be followed up with chest CT during short-term intervals. In these cases, preoperative chest CT could be used as a baseline study for follow-up. However, the large number of insignificant lesions detected on pre-operative chest CT may confuse the treatment strategy.
Figures and Tables
Fig. 1
Classification of the radiologic findings. (A) Normal. (B) Benign. (C) Metastatic. (D) Indeterminate.
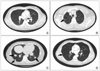
Fig. 2
Characteristics of the chest computed tomography (CT) group. F/U, follow-up. a)Thymoma (1), thyroid ca (1). b)Size <5 mm, without calcification.
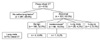
Fig. 3
Characteristics of the chest radiography (CXR) group. AP-CT, abdominopelvic-computed tomography.
References
1. McCormack PM, Attiyeh FF. Resected pulmonary metastases from colorectal cancer. Dis Colon Rectum. 1979. 22:553–556.
2. Ohlsson B, Pålsson B. Follow-up after colorectal cancer surgery. Acta Oncol. 2003. 42:816–826.
3. Blalock A. Recent advances in surgery. N Eng J Med. 1944. 231:261–267.
4. Thomford NR, Woolner LB, Clagett OT. The surgical treatment of metastatic tumors in the lungs. J Thorac Cardiovasc Surg. 1965. 49:357–363.
5. Inoue M, Ohta M, Iuchi K, Matsumura A, Ideguchi K, Yasumitsu T, et al. Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg. 2004. 78:238–244.
6. Rena O, Casadio C, Viano F, Cristofori R, Ruffini E, Filosso PL, et al. Pulmonary resection for metastases from colorectal cancer: factors influencing prognosis. Twenty-year experience. Eur J Cardiothorac Surg. 2002. 21:906–912.
7. Engstrom PF, Arnoletti JP, Benson AB 3rd, Chen YJ, Choti MA, Cooper HS, et al. Colon cancer. J Natl Compr Canc Netw. 2007. 5:884–925.
8. Engstrom PF, Arnoletti JP, Benson AB 3rd, Chen YJ, Choti MA, Cooper HS, et al. Rectal cancer. J Natl Compr Canc Netw. 2007. 5:940–981.
9. Kauczor HU, Hansen M, Schweden F, Strunk H, Mildenberger P, Thelen M. Computerized tomography in diagnosis of lung metastases: improvement with the spiral technique. Radiologe. 1994. 34:569–575.
10. Yun SH, Park SB, Kang SJ, Park CM, Jeong KW, Chang WY, et al. Is routine chest X-ray useful in detection of pulmonary metastases after curative resection for colorectal carcinoma? J Korean Soc Coloproctol. 2004. 20:169–175.
11. Viggiano RW, Swensen SJ, Rosenow EC 3rd. Evaluation and management of solitary and multiple pulmonary nodules. Clin Chest Med. 1992. 13:83–95.
12. Erasmus JJ, Connolly JE, McAdams HP, Roggli VL. Solitary pulmonary nodules: Part I. Morphologic evaluation for differentiation of benign and malignant lesions. Radiographics. 2000. 20:43–58.
13. Moore KH, McCaughan BC. Surgical resection for pulmonary metastases from colorectal cancer. ANZ J Surg. 2001. 71:143–146.
14. Goya T, Miyazawa N, Kondo H, Tsuchiya R, Naruke T, Suemasu K. Surgical resection of pulmonary metastases from colorectal cancer. 10-year follow-up. Cancer. 1989. 64:1418–1421.
15. Baron O, Amini M, Duveau D, Despins P, Sagan CA, Michaud JL. Surgical resection of pulmonary metastases from colorectal carcinoma. Five-year survival and main prognostic factors. Eur J Cardiothorac Surg. 1996. 10:347–351.
16. McCormack PM, Burt ME, Bains MS, Martini N, Rusch VW, Ginsberg RJ. Lung resection for colorectal metastases. 10-year results. Arch Surg. 1992. 127:1403–1406.
17. Irshad K, Ahmad F, Morin JE, Mulder DS. Pulmonary metastases from colorectal cancer: 25 years of experience. Can J Surg. 2001. 44:217–221.
18. Zink S, Kayser G, Gabius HJ, Kayser K. Survival, disease-free interval, and associated tumor features in patients with colon/rectal carcinomas and their resected intra-pulmonary metastases. Eur J Cardiothorac Surg. 2001. 19:908–913.
19. McAfee MK, Allen MS, Trastek VF, Ilstrup DM, Deschamps C, Pairolero PC. Colorectal lung metastases: results of surgical excision. Ann Thorac Surg. 1992. 53:780–785.
20. Povoski SP, Fong Y, Sgouros SC, Kemeny NE, Downey RJ, Blumgart LH. Role of chest CT in patients with negative chest x-rays referred for hepatic colorectal metastases. Ann Surg Oncol. 1998. 5:9–15.
21. Nakagawa K, Matsubara T, Tsuchiya S, Kinoshita I. Early detection and differential diagnosis of metastatic lung tumor. Gan No Rinsho. 1988. 34:1467–1477.
22. Ike H, Shimada H, Ohki S, Togo S, Yamaguchi S, Ichikawa Y. Results of aggressive resection of lung metastases from colorectal carcinoma detected by intensive follow-up. Dis Colon Rectum. 2002. 45:468–473.
23. Schoemaker D, Black R, Giles L, Toouli J. Yearly colonoscopy, liver CT, and chest radiography do not influence 5-year survival of colorectal cancer patients. Gastroenterology. 1998. 114:7–14.
24. Lee WS, Yun SH, Chun HK, Lee WY, Yun H. Linical usefulness of chest radiography in detection of pulmonary metastases after curative resection for colorectal cancer. World J Surg. 2007. 31:1502–1506.
25. Anthony T, Simmang C, Hyman N, Buie D, Kim D, Cataldo P, et al. Practice parameters for the surveillance and follow-up of patients with colon and rectal cancer. Dis Colon Rectum. 2004. 47:807–817.
26. Murata K, Takahashi M, Mori M, Kawaguchi N, Furukawa A, Ohnaka Y, et al. Pulmonary metastatic nodules: CT-pathologic correlation. Radiology. 1992. 182:331–335.
27. Kronawitter U, Kemeny NE, Heelan R, Fata F, Fong Y. Evaluation of chest computed tomography in the staging of patients with potentially resectable liver metastases from colorectal carcinoma. Cancer. 1999. 86:229–235.
28. Scholten ET, Kreel L. Distribution of lung metastases in the axial plane. A combined radiological-pathological study. Radiol Clin (Basel). 1977. 46:248–265.
29. Müller KM, Respondek M. Pulmonary metastases: pathological anatomy. Lung. 1990. 168:Suppl. 1137–1144.




 Citation
Citation Print
Print





 XML Download
XML Download