Abstract
Purpose
This study aimed to investigate early and mid-term outcomes of carotid artery stenting (CAS).
Methods
We retrospectively reviewed 111 patients who were treated for carotid stenosis between October 2004 and December 2009 (42 CASs and 69 carotid endarterectomies [CEAs]).
Results
CAS group was older than CEA group (70 years vs. 67 years, P = 0.001). Coronary artery disease and high lesion above the 2nd cervical vertebral body were more common in CAS group (29% vs. 13%, P = 0.002; 4% vs. 24%, P = 0.004). The 30-days stroke rate was higher in CAS group (10% vs. 1% in CEA group, P = 0.067, Fisher's exact test). New brain lesions on diffusion-weighted magnetic resonance imaging were more common in CAS group (48% vs. 20% in CEA group, P = 0.002, chi-square test). The 1-, 3-year freedom from stroke were 91%, 84% in CAS group and 99%, 99% in CEA group (P = 0.007, log-rank test). Univariate analysis showed that female gender and age > 70 years were related with postprocedural neurological complications (P = 0.046 and P = 0.007, log-rank test). However, none were independent risk factors on multivariate analysis.
Through large randomised controlled trials (RCTs), carotid endarterectomy (CEA) has been positioned as a gold standard of treatment in patients with carotid artery stenosis to prevent ischemic stroke [1-4]. Along with the advancement of endovascular treatment, carotid artery stenting (CAS) was introduced as a potential alternative to CEA during the past two decades. Possible benefits of CAS are less invasiveness (available in patients with high-risk morbidity, quicker recovery, lower wound complication), lower incidence of cranial nerve injury, coverage of entire length of carotid lesion, feasible in inaccessible lesion by CEA, feasibility in hostile neck (prior neck surgery or radiation) [5].
Among several RCTs comparing CEA vs. CAS in patients with atherosclerotic carotid disease, some reported the non-inferiority of CAS to CEA [6], while others failed to prove it [7,8]. And some studies are still ongoing [9-11]. Therefore, current practice guidelines recommend CAS as a possible alternative treatment to CEA only in patients with severe carotid stenosis and high operative risk [12,13].
In this study, we aimed to investigate early and mid-term outcomes of CAS in our hospital and to compare them with those of CEA. And we attempted to determine the risk factors of stroke after CAS.
Between October 2004 and October 2009, 113 patients with atherosclerotic carotid artery stenosis underwent primary carotid artery intervention. One hundred eleven patients were enrolled in this study excluding 2 patients who underwent emergency CAS and thrombolytic therapy of the middle cerebral artery occlusion. The indications of surgical or endovascular treatment were: 1) >70% asymptomatic carotid artery stenosis, 2) >80% asymptomatic stenosis, and 3) >50% stenosis with ulcerative plaque. Carotid stenosis percentage was determined angiographically according to North American Symptomatic Carotid Endarterectomy Trial criteria. Regardless of the type of ulcer, ulcerative plaque was diagnosed by 2 radiologists if there was evidence, on at least 1 angiographic view, that was considered likely to be an ulcer. During pre-treatment evaluation, all patients were categorized into high or low risk group. High risk was defined as clinically significant cardiac disease (congestive heart failure, unstable angina or need for urgent heart surgery), severe pulmonary disease, high lesion above the 2nd cervical vertebral body, previous radical neck surgery or ipsilateral carotid surgery, previous cervical radiation, contralateral recurrent laryngeal nerve palsy, presence of a tracheostomy stoma, contralateral carotid occlusion. For high-risk group, CAS was preferred. CAS was performed in 42 patients (CAS group, 38%) and CEA was done in 69 patients (CEA group, 62%).
During CAS, predilatation was done in all patients. Brain protection device (BPD; FilterWire EZ system, Boston Scientific Co., Natick, MA, USA) was used in 41 patients (98%). Self-expendable stents were inserted in all cases; Closed-cell type (WALLSTENT, Boston Scientific Co.) was 22 (52%) and open cell type (PRECISE, Cordis Co., Hialeah, FL, USA) was 20 (48%).
All CEA was performed under general anesthesia and selective shunting using a Pruitt-Inahara carotid shunt (Number 2004-49; LeMaitre Vascular Inc., St. Petersbur, FL, USA). A carotid shunt was used if >50% decrease in intraoperative electroencephalography amplitude and/or transcranial Doppler shows >50% decrease in mean flow velocity of the ipsilateral middle cerebral artery when the internal carotid artery (ICA) was clamped. Eversion endarterectomy was done in 7 patients and conventional technique with primary closure was used for other patients.
Asprin (100 mg/day) and clopidogrel (75 mg/day) was prescribed for CAS patients from 3 days before treatment and CEA patients received aspirin (100 mg/day) alone. After treatment both groups received aspirin and clopidogrel.
Postoperative neurologic evaluation was performed by a neurologist and diffusion-weighted brain magnetic resonance imaging (DW-MRI) was checked within a week. Clinical follow-up including duplex scans were done at 1 month, 3 months, 6 months, and 12 months for the first year after treatment and annually thereafter on the basis of out-patient clinic by neurologists and vascular surgeons.
On the duplex scan, restenosis was defined as a 50% diameter reduction of ICA combined with ICA peak systolic velocity greater than 125 cm/sec. If restenosis was detected, further angiographic evaluation was indicated. Patient database and medical records were reviewed retrospectively.
Patient demographic data, early (≤30 days) and late (>30 days) complications and outcomes including hematoma, cranial nerve injury, hyperperfusion syndrome, intracranial hemorrhage, stroke, myocardiac infarction and death were identified. Patient demographic data and early outcomes were compared between CAS and CEA group by t-test, chi-square test and Fisher's exact test. Freedom from ipsilateral stroke was calculated by Kaplan-Meier method and compared by log-rank test. Multivariate analysis using Cox proportional hazard model was conducted to determine the risk factors of stroke after CAS.
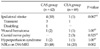
Patient demographic and clinical data are demonstrated in Table 1. CAS group was older than CEA group (mean age, 70 vs. 66; P = 0.001, t-test) and coronary artery disease was more common in CAS group (29% vs. 13%, P = 0.043, chi-square test). There was no difference in other variables between the 2 groups. The mean follow-up duration was 25 months (range, 1 to 70 months).
Table 2 demonstrates early phase (≤ 30 days) outcomes. 30-day stroke rate was inferior in CAS group to CEA group, however it was not statistically significant (10% vs. 1%, P = 0.067, Fisher's exact test). Postoperative DW-MRI revealed new brain lesions (NBLs) in 48% of CAS group while in 20% of CEA group (P = 0.002, chi-square test). There was no 30-day mortality in both groups.
Regarding mid-term outcomes, 1-year, 3-year freedom from ipsilateral stroke was 90%, 83% in CAS group and 99%, 99% in CEA group (P = 0.007, log-rank test, Fig. 1) and 1-year, 3-year freedom from restenosis was 97%, 86% in CAS group and 96%, 89% in CEA group (P = 0.454, log-rank test, Fig. 2). Target lesion revascularization was done in 3 patients of CAS group and 4 patients of CEA group during the follow-up.
The univariate analyses between stroke negative group and stroke positive group was done with variables such as, age, sex, smoking, hypertension, diabetes mellitus, coronary arterial disease, chronic pulmonary disease, cerebrovascular disease, hypercholesterolemia, plaque status, amount of stenosis, level of stenosis, symptomatic or asymptomatic, stent type, cardiac risk factor, pulmonary risk factor, anatomical risk factor. The female gender and age > 70 years showed statistically significant differences (P = 0.046 and P = 0.007, log-rank test). However, no independent risk factor of ipsilateral stroke after CAS was identified on multivariate analysis.
The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) study showed promising results for CAS vs. CEA (12.2% vs. 20.1% in 1-year major adverse events, P = 0.004 for non-inferiority) [6]. Recently, authors reported no significant difference in 3-year outcomes between 2 groups, such as freedom from major adverse event, freedom from death and freedom from stroke [14].
However, the Endarterectomy versus Stenting in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial was terminated early for safety and futility reasons because the 30-day stroke or death was significantly higher in CAS group (9.6% vs. 3.9% in CEA group; relative risk [RR], 2.5; confidence interval [CI], 1.2 to 5.1) [7]. However, after up to 4-year follow-up, the risk of ipsilateral stroke was similar in both groups after periprocedural period [15]. The authors concluded that CAS is as effective as CEA for middle-term prevention of ipsilateral stroke, but the safety of CAS needs to be improved. The Stent-Supported Percutaneous Angioplasty of the Carotid Artery versus Endarterectomy (SPACE) trial also reported equivalent 2-year results of CAS compared with CEA even though it had failed to prove non-inferiority of CAS for the 30-day results [16]. CAS group also showed higher risk of stroke, death, or procedural myocardial infarction in the intention-to-treat analysis (8.5% vs. 5.2%; RR, 1.69; CI, 1.16 to 2.45) on short-term (≤ 120 days) results of the International Carotid Stenting Study that were recently reported [17].
In our study, perioperative stroke rates after CAS were higher than we expected (9% in asymptomatic patients, 10% in symptomatic patients and 10% in total patients). It was higher than CEA group, although it was not statistically significant (10% vs. 1%, P = 0.067). It is assumed that the small sample size caused type II error. However, after perioperative period, the Kaplan-Meier curves of CAS and CEA groups are almost parallel as in the EVA-3S and SPACE study. This means mid-term stroke risk is similar between 2 groups and CAS can be a potential alternative to CEA if perioperative complications of CAS are reduced.
One of the causes which might explain the high periprocedural stroke rate after CAS is plaque instability. During CEA, not only plaque morphology but also plaque natures are visualized: smooth wall vs. irregular wall, soft plaque vs. hard plaque, ulcerative lesion, calcified lesion and plaque with hemorrhage. The vulnerable plaque is generally described as having a lipid core with a fibrous cap with thinning of the cap and inflammation in the shoulder region of the plaque [18]. In particular, when CAS is performed on vulnerable plaque or ulcerative plaque, there will be a high risk of embolization. Although a BPD is used, the guide wire and the device should be passed the lesion before. In this study, early stoke occurred more often in patients with plaque ulcers than without, even though it failed to make a significant difference (17% vs. 7%, P = 0.067, Fisher's exact test). To identify a high-risk plaque, several techniques have been introduced. The Imaging in Carotid Angioplasty and Risk of Stroke study announced that carotid echolucency (the gray-scale median ≤ 25) increases the risk of stroke during CAS [19]. Other noble molecular imaging techniques, such as positron emission tomography, single photon emission computed tomography, near-infrared fluorescence, and high-resolution MRI are introduced [20]. They may helpful to select the candidates for CAS in the future, but currently, for further evidence, large clinical prospective trials are required.
After the SAPPHIRE trial, the United States (US) Food and Drug Administration approved the use of CAS for symptomatic patients who are considered high-risk surgical candidates. Use of CAS has been increasing since. Despite continuous effort and advancement in technology, early major adverse event rate is still high. Recent nation-wide data in the US demonstrated that CAS had increased rates of postprocedure stroke (1.8% vs. 1.1%, P < 0.05) and death (1.1% vs. 0.57%, P < 0.05) as opposed to CEA [21]. Some authors announced favourable outcomes after CEA even in "high-risk" patients [22-24]. One survey based on the American College of Surgeons National Surgical Quality Improvement Program database showed that CEA is associated with favourable 30-day outcomes across a spectrum of patient comorbidity features and only anatomic and technical features are independent predictors of perioperative stroke [25].
Another issue is NBLs after treatment. On meta-analysis of 32 studies, the incidence of any NBLs on DW-MRI was significantly higher after CAS than after CEA (37% vs. 10%, P < 0.01) [26]. And authors reported that the use of BPD (33% vs. 45% without, P < 0.01), closed-cell designed stents (31% vs. 51% with open-cell stent) during CAS and selective shunting (6% vs. 16% of routine shunting, P < 0.01) during CEA were protective factor of NBLs. In this study, CAS resulted in higher incidences of NBLs compared with CEA (53% vs. 24%, P = 0.002). However, the significance of microemboli is an unanswered question, currently. In a 2-year prospective study, 90% of the events were clinically silent [27].
A large prospective, population-based study demonstrated that elderly people with silent brain infarcts on MRI are associated with higher risk of dementia and a steeper decline in cognitive function [28]. However, there are few studies investigating the relation between external cranial carotid artery revascularization and neuropsychological dysfunction [29,30]. Regarding the effect on cognitive function, microembolism during procedure may induce adverse effects while possibly inducing positive effects by increasing brain perfusion. For this question, further study is needed.
The debate as to whether CAS is an alternative to CEA is ongoing. Our results showed unacceptable periprocedural stroke rates in CAS group. These results suggest that CEA should be the treatment of choice for patients with carotid artery stenosis and CAS should be performed with more restricted criteria in an experienced hospital. The limitations of this study are a retrospective design and small sample size.
Figures and Tables
Fig. 1
Kaplan-Meier curves comparing freedom from ipsilateral stroke between the carotid artery stenting (CAS) and carotid endarterectomy (CEA) groups.

Fig. 2
Kaplan-Meier curves comparing freedom from restenosis between the carotid artery stenting (CAS) and carotid endarterectomy (CEA) groups.

References
1. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998. 351:1379–1387.
2. Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998. 339:1415–1425.
3. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995. 273:1421–1428.
4. Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004. 363:1491–1502.
5. Narins CR, Illig KA. Patient selection for carotid stenting versus endarterectomy: a systematic review. J Vasc Surg. 2006. 44:661–672.
6. Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004. 351:1493–1501.
7. Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006. 355:1660–1671.
8. SPACE Collaborative Group. Ringleb PA, Allenberg J, Brückmann H, Eckstein HH, Fraedrich G, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006. 368:1239–1247.
9. Hobson RW 2nd, Howard VJ, Brott TG, Howard G, Roubin GS, Ferguson RD, et al. Organizing the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): National Institutes of Health, Health Care Financing Administration, and industry funding. Curr Control Trials Cardiovasc Med. 2001. 2:160–164.
10. Featherstone RL, Brown MM, Coward LJ. ICSS Investigators. International carotid stenting study: protocol for a randomised clinical trial comparing carotid stenting with endarterectomy in symptomatic carotid artery stenosis. Cerebrovasc Dis. 2004. 18:69–74.
11. Rudarakanchana N, Dialynas M, Halliday A. Asymptomatic Carotid Surgery Trial-2 (ACST-2): rationale for a randomised clinical trial comparing carotid endarterectomy with carotid artery stenting in patients with asymptomatic carotid artery stenosis. Eur J Vasc Endovasc Surg. 2009. 38:239–242.
12. Hobson RW 2nd, Mackey WC, Ascher E, Murad MH, Calligaro KD, Comerota AJ, et al. Management of atherosclerotic carotid artery disease: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2008. 48:480–486.
13. Liapis CD, Bell PR, Mikhailidis D, Sivenius J, Nicolaides A, Fernandes e Fernandes J, et al. ESVS guidelines. Invasive treatment for carotid stenosis: indications, techniques. Eur J Vasc Endovasc Surg. 2009. 37:4 Suppl. 1–19.
14. Gurm HS, Yadav JS, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008. 358:1572–1579.
15. Mas JL, Trinquart L, Leys D, Albucher JF, Rousseau H, Viguier A, et al. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 2008. 7:885–892.
16. Eckstein HH, Ringleb P, Allenberg JR, Berger J, Fraedrich G, Hacke W, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. 2008. 7:893–902.
17. International Carotid Stenting Study investigators. Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HB, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet. 2010. 375:985–997.
18. Fishbein MC. The vulnerable and unstable atherosclerotic plaque. Cardiovasc Pathol. 2010. 19:6–11.
19. Biasi GM, Froio A, Diethrich EB, Deleo G, Galimberti S, Mingazzini P, et al. Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. Circulation. 2004. 110:756–762.
20. Hermus L, van Dam GM, Zeebregts CJ. Advanced carotid plaque imaging. Eur J Vasc Endovasc Surg. 2010. 39:125–133.
21. McPhee JT, Schanzer A, Messina LM, Eslami MH. Carotid artery stenting has increased rates of postprocedure stroke, death, and resource utilization than does carotid endarterectomy in the United States, 2005. J Vasc Surg. 2008. 48:1442–1450.
22. Mozes G, Sullivan TM, Torres-Russotto DR, Bower TC, Hoskin TL, Sampaio SM, et al. Carotid endarterectomy in SAPPHIRE-eligible high-risk patients: implications for selecting patients for carotid angioplasty and stenting. J Vasc Surg. 2004. 39:958–965.
23. Boules TN, Proctor MC, Aref A, Upchurch GR Jr, Stanley JC, Henke PK. Carotid endarterectomy remains the standard of care, even in high-risk surgical patients. Ann Surg. 2005. 241:356–363.
24. Flanigan DP, Flanigan ME, Dorne AL, Harward TR, Razavi MK, Ballard JL. Long-term results of 442 consecutive, standardized carotid endarterectomy procedures in standard-risk and high-risk patients. J Vasc Surg. 2007. 46:876–882.
25. Kang JL, Chung TK, Lancaster RT, Lamuraglia GM, Conrad MF, Cambria RP. Outcomes after carotid endarterectomy: is there a high-risk population? A National Surgical Quality Improvement Program report. J Vasc Surg. 2009. 49:331–338. 339.e1
26. Schnaudigel S, Gröschel K, Pilgram SM, Kastrup A. New brain lesions after carotid stenting versus carotid endarterectomy: a systematic review of the literature. Stroke. 2008. 39:1911–1919.
27. Hammer FD, Lacroix V, Duprez T, Grandin C, Verhelst R, Peeters A, et al. Cerebral microembolization after protected carotid artery stenting in surgical high-risk patients: results of a 2-year prospective study. J Vasc Surg. 2005. 42:847–853.
28. Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003. 348:1215–1222.
29. Heyer EJ, DeLaPaz R, Halazun HJ, Rampersad A, Sciacca R, Zurica J, et al. Neuropsychological dysfunction in the absence of structural evidence for cerebral ischemia after uncomplicated carotid endarterectomy. Neurosurgery. 2006. 58:474–480.
30. Grunwald IQ, Supprian T, Politi M, Struffert T, Falkai P, Krick C, et al. Cognitive changes after carotid artery stenting. Neuroradiology. 2006. 48:319–323.




 Citation
Citation Print
Print



 XML Download
XML Download