Abstract
Objectives
Menopause is the permanent cessation of menstruation resulting from the loss of ovarian follicular activity. There is limited and conflicting evidence for an association between lung function and menopause. The purpose of this study is to evaluate Pulmonary Function Test (PFT) in postmenopausal women.
Menopause (“end of monthly cycles”) is a major but normal biological event in a woman's life. All sex steroid hormone receptors are expressed in lung tissue. It is associated with marked reduction in levels of estradiol and has important effects on female physiology. Menopause has been associated with increased respiratory disorders.1
Pulmonary Function Tests (PFTs) provide objective, quantifiable data of pulmonary function and spirometry tests based on voluntary forced expiration can detect changes in respiratory system.
Literature on lung health in women is confusing and effects of hormonal status on the airways often appear to be heterogenous.2 Aim of the present study was to evaluate the effect of menopause on PFTs.
Present work was undertaken in the Department of Physiology, Government Medical College, Jammu, India over a span of one year. The project was approved by the Institutional Ethics Committee. The subjects enrolled were outpatients from Department of Gynaecology and Obstetrics, Government Medical College, Jammu and those who volunteered to join the study. Their general physical examination and clinical examination of respiratory system were performed to determine the eligibility. A written informed consent was taken from all eligible subjects.
Exclusion criteria: (1) history of hypertension; diabetes mellitus; (2) heart disease; (3) tuberculosis; (4) asthma; (5) occupational lung disease; and (6) chronic obstructive pulmonary disease subjects with rhinitis, common cold, dyspnea or cough.
Total 115 women were screened and 95 met the eligibility criteria. Depending on their menstrual history, the subjects were divided into two groups: Group I (n = 49) comprised healthy premenopausal women (with regular menstrual cycles) with lower age limit of 40 years. Group II (n = 46) had healthy postmenopausal women (defined as those women who did not have any menstruation for past 12 months) with upper age limit of 55 years.
Age of the eligible subjects was recorded in years as per their statement Body Weight in kg and the height was measured in centimetres. Body surface area (BSA) was calculated in square meters from Dubois Nomogram.
A portable, computerized spirometer (DTSpiro; Maestro Medline. System Ltd, Himachal Pradesh, India) was used and best of three readings was considered. The parameters recorded were: forced vital capacity (FVC); forced expiratory volume in half second (FEV0.5); FEV in first second (FEV1); FEV in 3 seconds (FEV3); peak expiratory flow rate (PEFR); mean forced expiratory flow (FEF) during middle half of FVC (FEF25-75%); FEF 25% (FEF25%); FEF 50% (FEF50%); FEF 75% (FEF75%); FEV0.5/FVC, FEV1/FVC, FEV3/FVC; maximum voluntary ventilation (MVV); and FVC time (FVC time). Acceptability criteria were as per Miller.3
For better understanding of the observed PFT values, the software installed in the spirometer also calculates and displays the predicted values of PFTs at the same time as actual values. These predicted values are derived from average values of a large population of healthy subjects.
The data was analysed by SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Differences in mean were tested using Student's t-test. A P-value < 0.05 was considered statistically significant.
Mean age of the women in postmenopausal group was 51 ± 2.49 years; mean body weight and BSA were higher in these subjects (Table 1). Mean height was comparable in the two groups.
Comparison of PFT parameters and flow rates between two groups showed reduced values in Group II subjects (Table 2, 3). Mean observed values of FVC, FEV0.5, FEV1, FEV0.5/ FVC, FEV1/FVC were significantly reduced whereas FVC time was prolonged in the postmenopausal group (Table 2). Difference between the two groups was not significant for FEV3 and FEV3/FVC.
All flow rate parameters viz. PEFR, FEF25-75%, FEF25%, FEF50%, FEF75% and MVV (L/min) were observed to be reduced in postmenopausal women (Table 3).
Percentage of predicted values of all observed Spirometric parameters in the two groups were compared to exclude the effects of age and body mass index (BMI) on PFTs and were found to be in line with the other findings of our study except for FEF75% (Fig. 1).
There is limited information on potential changes in respiratory health when women enter the menopausal state and the effect of menopause on decline of pulmonary functions has not been investigated in Indian population to the best of our knowledge.
Menopause is permanent cessation of ovarian follicular activity and eventually, the menstrual cycle. Reduced levels of estrogen and progesterone could cause decreased muscular strength, decreased relaxation of bronchial smooth muscle and increased compression of thoracic spine due to osteoporosis.
Measurement of lung volumes and capacities is considered a key indicator of physical health. PFTs provide quantitative measures of various bronchopulmonary functions by which we define the normal values and also determine the nature and extent of bronchopulmonary dysfunctions.4
Mean age of Group II (postmenopausal women) was 51 ± 2.49 years. This finding is similar to study done by Kumar et al.5. Average age of menopause in western world is 51 years.6 However, studies emanating from our country have revealed the average age of menopause in India to range from 43.55 to 49.35 years.789101112
Mean body weight of postmenopausal women was more (P = 0.001) and could be attributed to declining estrogen levels.
Mean height was similar in both groups (P = 0.719) but mean BSA of postmenopausal women was significantly higher (P = 0.001). This increase could be attributed to increase in mean body weight of the postmenopausal women.
Postmenopausal women not only experience physical effects of aging but physiological aspects like lung function are also impacted, the cause being lack of ovarian hormones. There is very less literature regarding effect of menopause on lung functions, even worldwide. We sought to investigate whether the menopausal status is related to the lung health.
FVC is a direct function of the amount of inhaled air. It is the most important PFT,13 very sensitive to diseases that affect the lung elasticity and mechanical properties. A low FVC may be caused by either obstruction or restriction.
In the present study, mean FVC was significantly less in Group II. Our findings agree with Strinic13, Real et al.14, Polly et al.15, Hayatbakhsh et al.16 and Triebner et al.17.
Impaired lung function, as measured by FVC or FEV1 has been indicated as a marker of premature death Young et al.18.
It indicates the amount of air exhaled with maximum effort in the first half second of FVC maneuver and is a better indicator of pulmonary ventilation.
In our work, significantly less values of FEV0.5 in Group II were observed as compared to those of premenopausal women (P < 0.0001). A search of the literature revealed no work related to this parameter.
Not only is FEV1 (normal value 80%) the most widely used spirometric parameter, it remains the single most validated and clinically useful test of ventilatory function for assessment and management of airflow limitation. FEV1 is a universally reliable indicator in distinguishing between obstructive and restrictive lung diseases.
In the present study, the observed mean values of FEV1 of postmenopausal women were significantly less as compared to those of premenopausal women (P < 0.0001). The results of our study agree with Strinic13, Real et al.14, Polly et al.15, Hayatbakhsh et al.16 and Jung et al.20.
Observed mean FEV1 as percentage of predicted value in the premenopausal women was significantly better than in postmenopausal group (124.85 vs 72.40%, P < 0.0001). According to Pata et al.21, and Cevrioglu et al.22, lower percentage of predicted values of FEV1 in postmenopausal women in comparison to premenopausal women are likely due to decrease level of progesterone and estrogen.
Our findings are in disagreement with Triebner et al.17, and this contradiction could be due to differences in anthropometric measurements, environmental and geographical conditions.
In the present study, the observed mean values of FEV3 of postmenopausal women were comparable with those of premenopausal women (P = 0.191). No report has been cited in the literature regarding effect of menopause on this parameter.
FEV0.5/FVC (%), FEV1/FVC (%), FEV3/FVC (%) are additional parameters for information on ventilatory functions.
The observed mean values of FEV0.5/FVC of postmenopausal women were significantly less as compared to those of premenopausal women (P < 0.0001).
Review of literature revealed no work in context of this parameter.
The amount of air exhaled during the first second is a fairly constant fraction of the FVC irrespective of lung size. Generally expressed as a percentage, in normal adults, this ratio ranges from 75% to 85%.13
FEV1/FVC ratio is a much more useful index than FVC alone and allows separation of patients with ventilator abnormalities into obstructive or restrictive type.
In the present study, mean value of FEV1/FVC of postmenopausal women was significantly less as compared to those of premenopausal women (45.79% vs. 70.10%, P < 0.0001) indicating obstructive pattern. Also, FEV1/FVC as percentage of predicted value in premenopausal women was significantly better as compared to that of postmenopausal women (93.24% vs. 62.15%, P < 0.0001).
Polly et al.15 found the mean FEV1/FVC value to be higher but statistically non-significant in postmenopausal women.
Strinic14 found no decrease in FEV1/FVC in postmenopausal women. Difference between the two studies in terms of ethnicity, anthropometry, environmental and geographical conditions of subjects taken may have caused our study results to be different.
Subjects with reduced FEV3/FVC have a progressively increased risk of developing obstruction.23
In the present study, the observed mean values of FEV3/FVC of postmenopausal women were comparable with those of premenopausal women (P = 0.619).
Regarding the effect of menopause on this parameter, no report could be found in the literature.
PEFR is a method of assessing the ventilatory capacity with single breath. It is largely a function of the caliber of large airways. It is abnormally decreased only in moderate to severe airway obstruction.24 Important advantage of peak flow rate lies in the fact that it is relatively easily understood by the subject and physiologically a well-defined index.
In the present study, the observed mean value of PEFR was significantly less in postmenopausal women as compared to premenopausal women (P < 0.0001).
Strinic13, Polly et al.15, Kumar et al.5 also observed similar reduction in PEFR in postmenopausal women.
Such a decline, according to Kumar et al.5, is due to two reasons. First, due to aging and second due to decreased level of progesterone. Pulmonary changes due to aging are decrease in lung compliance with increased rigidity, lowered diaphragm and loss of internal alveolar surface area. Residual volume increases in aging due to less vigorously ascent of diaphragm and decreased elastic coil.
Decrease in progesterone level alters the contractility of smooth muscle and increases the hydration of airway mucosa because progesterone is important in regulation of microvascular leakage in airways.25
Expiratory flow rates are additional indices of airway resistance besides FEV1. None of these have any particular advantage over FEV1 except FEF25-75% which is less effort dependent and reflects the behaviour of small as well as large airways.26 These flow rates are being used increasingly to assess the lung function in early diagnosis of airway obstruction especially when peripheral airways are involved.
FEF25-75% reflects effort independent expiration and the status of small airways.
In the present study, the mean values of FEF25-75% of postmenopausal women were significantly less as compared to those of premenopausal women (P < 0.0001).
Mean FEF25% of postmenopausal women was significantly less than in premenopausal women (P < 0.0001).
No report could be found in the literature regarding effect of menopause on this parameter.
In the present study, mean values of FEF50% of postmenopausal women were significantly less as compared to those of premenopausal women (P < 0.0001). The results of our study agree with Strinic.14
In our study, mean values of FEF75% in postmenopausal women were significantly less than in premenopausal counterparts (0.72 vs. 1.01 L/sec; P < 0.0008). However for this one parameter, when the observed values were compared to predicted values for two groups, the difference between them was not significant (101.24 vs. 95.30%; P = 0.516). This can be attributed to factors like age and body size/BMI of our subjects because other factors which could vary the predicted values e.g. gender, race and altitude are same in two groups.
Our results agree with Strinic13.
MVV is the largest volume of air that can be moved into and out of the lungs in one minute by voluntary effort. It is a dynamic test of lung function and is considered to be a good guideline of the mechanical efficiency of lungs. However, MVV is less sensitive than FEF25-75%.
In our study, mean MVV in postmenopausal women was significantly less as compared to premenopausal women (P < 0.0001)
Review of literature revealed no work in context of this parameter.
Some obstructed patients have a relatively normal FVC in relation to their predicted values. However, the time required to expire their FVC is usually prolonged.27
In the present study, the FVC time (min) taken by postmenopausal women was significantly more as compared to that premenopausal women (P = 0.006).
A search of available literature failed to show any report on this parameter.
Primary purpose of PFT is to identify the severity of pulmonary impairment. Loss of lung function occurs quickly in postmenopausal women.2728 Estrogen along with progesterone has been associated with airway smooth muscle relaxation which reduces the contractile response of these respiratory muscles.293031 Estrogen could play a crucial role in collagen production.32
Results from our study suggest that women tend to develop obstructive pattern of ventilatory dysfunction and will add to the data on biological role of the ovarian hormones in modulating airways. Specific health education strategies have to be made to deal with the health problems faced by women after menopause.
Figures and Tables
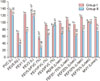 | Fig. 1Comparison of pulmonary function tests as percentage of predicted values between subjects of group I and group II. *Significant P < 0.00. †Highly significant P < 0.0001. ‡No significant, P > 0.05. |
References
2. Macsali F, Svanes C, Bjorge L, Omenaas ER, Gomez Real F. Respiratory health in women: from menarche to menopause. Expert Rev Respir Med. 2012; 6:187–200.

3. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005; 26:319–338.
. Slonim NB, Chapin JL. Respiratory physiology. St. Louis, MO: Mosby;1967.
5. Kumar S, Shah C, Oommen ER. Study of cardiovascular risk factors In pre and postmenopausal women. Int J Pharma Sci Res. 2012; 3:560–570.
6. Bharadwaj JA, Kendurkar SM, Vaidya PR. Age and symptomatology of menopause in Indian women. J Postgrad Med. 1983; 29:218–222.
7. Randhawa I, Premi HK, Gupta T. The age at menopause in the women of Himachal Pradesh, and the factors affecting the menopause. Indian J Public Health. 1987; 31:40–44.
8. Shah R, Kalgutkar S, Savardekar L, Chitlang S, Iddya U, Balaiah D. Menopausal symptoms in urban Indian women. Obstet Gynecol Today. 2004; 11:667–670.
9. Singh A, Arora AK. Profile of menopausal women in rural north India. Climacteric. 2005; 8:177–184.

10. Sharma S, Tandon VR, Mahajan A. Menopausal symptoms in urban women. JK Sci. 2007; 9:13–17.
11. Tandon VR, Mahajan A, Sharma S, Sharma A. Prevalence of cardiovascular risk factors in postmenopausal women: A rural study. J Midlife Health. 2010; 1:26–29.

12. Hyatt RE, Schilder DP, Fry DL. Relationship between maximum expiratory flow and degree of lung inflation. J Appl Physiol. 1958; 13:331–336.

13. Strinic T. Disorders of pulmonary ventilation function in patients with genital prolapse in postmenopause. Lijec Vjesn. 1999; 121:78–81.
14. Real FG, Svanes C, Omenaas ER, Anto JM, Plana E, Jarvis D, et al. Lung function, respiratory symptoms, and the menopausal transition. J Allergy Clin Immunol. 2008; 121:72–80.e3.
15. Polly ZA, Begum S, Ferdousi S, Begum N, Ali T, Begum A. Relationship of FEF25-75, pefr and SVC with estrogen and progesterone level in postmenopausal women. J Bangladesh Soc Physiol. 2011; 6:116–121.

16. Hayatbakhsh MR, Najman JM, O'Callaghan MJ, Williams GM, Paydar A, Clavarino A. Association between smoking and respiratory function before and after menopause. Lung. 2011; 189:65–71.

17. Triebner K, Matulonga B, Johannessen A, Suske S, Puggini L, Aymerich JG, et al. Menopause is associated with accelerated lung function decline in the longitudinal European community respiratory health survey. Eur Respir J. 2016; 48:OA4998.

18. Young RP, Hopkins R, Eaton TE. Forced expiratory volume in one second: not just a lung function test but a marker of premature death from all causes. Eur Respir J. 2007; 30:616–622.

19. Songur N, Aydin ZD, Ozturk O, Sahin U, Khayri U, Bircan A, et al. Respiratory symptoms, pulmonary function, and reproductive history: Isparta Menopause and Health Study. J Womens Health (Larchmt). 2010; 19:1145–1154.
20. Jung WJ, Kim YS, Jung JY, Jung KS, Kim EY, Kim SK, et al. The effect of menopause on the lung function among Korean women: the fourth Korean National Health and Nutrition Examination Survey (KHANES IV). Eur Respir J. 2012; 40:P3965.
21. Pata O, Atis S, Utku Oz A, Yazici G, Tok E, Pata C, et al. The effects of hormone replacement therapy type on pulmonary functions in postmenopausal women. Maturitas. 2003; 46:213–218.

22. Cevrioglu AS, Fidan F, Unlu M, Yilmazer M, Orman A, Fenkci IV, et al. The effects of hormone therapy on pulmonary function tests in postmenopausal women. Maturitas. 2004; 49:221–227.

23. Hegab S, Yessayan L, DiGiovine B, Morris ZQ. Prognostic significance of a reduced FEV3/FVC ratio. Am J Respir Crit Care Med. 2015; 191:A4469.
24. Murray JF, Mason RJ, Boushey HA, Nadel JA. Textbook of respiratory medicine. 3rd ed. Philadelphia, PA: Saunders;2000.
25. Beynon HL, Garbett ND, Barnes PJ. Severe premenstrual exacerbations of asthma: effect of intramuscular progesterone. Lancet. 1988; 2:370–372.

26. Hinshaw HC, Murray JF. Disease of the chest. 4th ed. London, UK: W.B. Saunders;1980.
27. Mottram C. Ruppel's manual of pulmonary function testing. 10th ed. St. Louis, MO: Mosby;2013.
28. Massaro D, Massaro GD. Toward therapeutic pulmonary alveolar regeneration in humans. Proc Am Thorac Soc. 2006; 3:709–712.

29. Woolcock J, Macklem PT, Hogg JC, Wilson NJ. Influence of autonomic nervous system on airway resistance and elastic recoil. J Appl Physiol. 1969; 26:814–818.

30. Raz S, Zeigler M, Caine M. The effect of progesterone on the adrenergic receptors of the urethra. Br J Urol. 1973; 45:131–135.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download