Abstract
Objectives
Atrophic vaginitis (AV), which is common in postmenopausal women, is characterized by vaginal dryness, dyspareunia, and discomfort. There are a variety of therapeutic agents for the treatment of AV, besides hormone replacement therapy. We performed this systematic review to compare the effectiveness of various therapies for symptom improvement in AV patients.
Methods
We searched the Cochrane Library, EMBASE, MEDLINE, and other literature (Google Scholar, Web of Science, and hand search) for studies published between January 2010 and March 2015. AV was evaluated by the following outcomes: vaginal pH, dyspareunia, vaginal dryness, or cytological change (endometrial thickness, percentages of superficial cells and parabasal cells). They measured treatment efficacy with various outcomes pertaining to AV symptoms.
Atrophic vaginitis (AV) appears in approximately 45% of postmenopausal women. Symptoms of AV are dyspareunia, dryness, changed vaginal pH, and urinary and genital changes.1 The decrease in serum estrogen level after menopause causes these symptoms. AV has potentially negative effects on quality of life and can be related to secondary diseases such as urinary tract infection.234
AV is treated with hormone replacement therapy (HRT), typically estrogen.56 Estrogen therapy is sufficient to restore normal vaginal acidity and microorganisms, assists epithelial thickening, causing an increase in vaginal discharge, and helps to improve the symptoms of AV.5 One approach to low-dose estrogen therapy is to insert estrogen or estradiol as a cream, tablet, or ring into the vagina. Systemic side effects of this therapy are possible, but the risk is insignificant.7 Potential risks of low-dose estrogen therapy have not been reported in a well-designed study. However, using this therapy for patients who form estrogen-dependent tumors, such as breast cancer survivors or patients with latent breast cancer, is controversial.8
Recently, in addition to estrogen, there has been interest in other treatments for AV, such as selective estrogen receptor modulators (SERM) (e.g., ospemifene), tissue-selective estrogen complexes (TSEC) (e.g., bazedoxifene), estriol, and natural products (e.g., soy isoflavone, sea buckthorn oil). SERM and TSEC, when combined with estrogen treatments, improve clinical benefits because they provide a specific estrogen receptor. Recently, some clinical trials have shown that TSEC containing bazedoxifene with estrogen treatment had effects on endometrial changes, decreased menopausal symptoms, and decelerated postmenopausal loss of bone mass.910 Estriol is a type of estrogen; tablets containing a low dose of estriol have been promoted for maturation and proliferation of the vaginal epithelial layer. A beneficial profile of the tablet has been indicated in diverse small clinical studies.11 Natural product treatments for AV that have been studied include soy isoflavone and sea buckthorn oil. Soy isoflavone has effects like those of estrogen, and it occurs naturally in soy.12 Sea buckthorn oil has also been studied because of associated improvement in the vaginal epithelium.13 In other study of AV, lipofilling with platelet-rich plasma (PRP) relieved symptoms. These are show that lipofilling with PRP can be effective for vaginal atrophy.14
A variety of therapies for AV are available, but it is hard to determine which therapies are most effective. In addition, it is need to think about the effects on AV symptoms.
This review was conducted to perform a network meta-analysis of randomized controlled trials (RCTs) to identify effective drugs for relief of AV and to identify drug efficacy on vaginal symptoms (vaginal pH, dyspareunia, vaginal dryness, and cytological change) in postmenopausal women.
This review was done with recommended methods according to PRISMA and MOOSE guideline. The databases were searched for publications that described RCTs of AV for the relief of menopausal vaginal symptoms. The primary outcome was the efficacy of drugs for treatment of AV; the secondary outcome was drug treatment efficacy for vaginal symptoms. To be eligible for inclusion, studies had to focus on AV and assess of the following outcomes: vaginal pH, dyspareunia, vaginal dryness, endometrial thickness, and change in the percentages of superficial and parabasal cells. Studies of bazedoxifene, bazedoxifene with conjugated estrogens, ospemifene, estriol, sea buckthorn oil, and soy isoflavone were included. Furthermore, reports had to provide adequate information to compare outcomes between treated groups and baseline in RCT. Articles satisfying these criteria were separated into RCTs and uncontrolled clinical trial series. An RCT had to include a control group or groups with random distribution to intervention study and had to use either a crossover or parallel design.
The review included a search of Medline, OVID Medline, Web of Science, Scopus, National Guideline Clearinghouse, Cochrane Library, Google Scholar, Medical Treatment Guidelines, CMA Infobase, UK Clinical Guidelines, Guideline International Network, KoreaMed, KISS, NDSL, and KMbase databases, as well as a hand search of the pertinent journals published between January 2010 and March 2015 using the key words vagina atrophy, vaginal epithelium, epithelium, atrophy, vagina, vaginitis, atrophic vaginitides, “vaginitides, atrophic,” “vaginitis, atrophic,” drug therapy, therapeutics, HRT, estrogen replacement therapy, gonadal hormones, gonadal steroid hormones, menopause, postmenopause, postmenopausal period, and climacteric. In all searches, limits were set for MeSH (human) and language (English or Korean).
Selection of study and extraction of date were independently performed by two investigators (ARL and BRL). Through consensus and discussion, discrepancies between the investigators were resolved. The final results were reviewed by the senior investigator (THK). Data extraction forms were used when necessary with appeal for provide missing data and the alternative to make corrections. Missing standard deviations were estimated from the range or the standard error of the mean.
Descriptive statistics were summarized for trial and study population characteristics across all the eligible trials, describing the types of comparisons and some important variables, either clinical or methodological (such as year of publication, age, and body mass index [BMI]).
We did a random-effects multiple-treatments meta-analysis within a Bayesian framework; the results are summarized using effect sizes and their credible intervals (CrI). The Bayesian network meta-analysis was carried out to make indirect comparisons among treatments when direct comparisons were not possible. Markov chain Monte Carlo simulation was used for the posterior distributions. Three parallel Markov chain Monte Carlo simulations were runned for a period of 10,000 iterations after the first 10,000 were deleted as the burn-in period. All the evidence was analyzed using two approaches: a consistency model and an inconsistency model. The fit of the two models was assessed using the deviance information criterion, and the model that showed lower residual deviance was selected.
We calculated the probability that each drug was the most effective (first best) drug, the second drug, the third drug, and so on; these results are presented graphically. The probability was the surface under the cumulative ranking (SUCRA) probabilities, which was expressed as percentages comparing each intervention to an imaginary intervention that is always the best without uncertainty. A SUCRA value of x percent means that the drug accomplishes x percent of the effectiveness of this imaginary drug; thus, larger SUCRA values denote more effective interventions.
All analyses were performed using R of version 3.1.3 (The R Foundation for Statistical Computing, Vienna, Austria) and WinBUGS version 1.4 (MRCBiostatistics Unit 2007, Cambridge, UK). If the difference between the variance of random-effects an inconsistency was large (P < 0.05), then significant heterogeneity was considered present.
For the study analysis, Fig. 1 displays the flow diagram. Of 99 potentially relevant articles initially screened, 9 trials was included by inclusion criteria in the final analysis. Six articles did not contain available data, 37 were review papers, 32 were not RCTs, 9 were not relevant to the topic, 4 were not suitable because of their study period, and 2 were not suitable for quantitative synthesis in the meta-analysis. Fig. 2 shows the evidence network. The available direct comparisons (i.e., intervention and placebo compared against each other in a RCT setting) are graphically depicted in the network graph. A node width is corresponding to the number of trials of drugs. Each node represents 1 drug. The data represented 13 pairwise comparisons (12 active drugs and the placebo treatment). Of the 12 direct comparisons, ospemifene vs. placebo was evaluated in 6 (50%) reports based on 2, 3, 8, and 9 tarils, respectively and 7 ofr two trials. Bazedoxifene plus conjugated estrogen vs. placebo was evaluated in 2 (16.7%) reports, each based on 1 trial. Other drugs (bazedoxifene, estriol vaginal gel, sea buckthorn oil, soy isoflavone vaginal gel) vs. placebo were each evaluated in 1 (8.3%) trial. The clinical characteristics of patients enrolled in the randomized trials are reported in Table 1 and 2. These studies included 4,034 patients with mean age of 58.3 years and mean BMI of 26.1 kg/m2. The duration of follow-up was 12 weeks for all studies. Study quality was acceptable in all cases.
Network meta-analysis combining direct and indirect estimates demonstrated that ospemifene 60 mg/day had the highest probability (55%) of being ranked as most effective and resulted in the greatest increase in the percentage of superficial cells (mean [95% CrI], 11.07 [−16.08, 37.83]). Results are summarized in Fig. 3A and 4A. Compared with the placebo, mean increases in the percentage of superficial cells were seen with bazedoxifene, bazedoxifene plus conjugated estrogen, and ospemifene, although these differences were not statistically significant. The intervention with bazedoxifene alone showed no results when compared with placebo.
Network meta-analysis combining direct and indirect estimates demonstrated that ospemifene 60 mg/day had the highest probability (27%) of being ranked as most effective and was associated with the greatest decrease in the percentage of parabasal cells (mean [95% CrI], −34.62 [−91.01, 20.90]). Ospemifene 30 mg/day and bazedoxifene 20 mg plus conjugated estrogen 0.625 mg also had comparable ranked probability (25% and 22%, respectively) and comparable mean decreases in the percentages of parabasal cells (mean [95% CrI], −21.28 [−128.50, 85.17] for osepemifene 30 mg; and mean [95% CrI], −22.13 [−141.70, 95.86] for bazedoxifene 20 mg plus conjugated estrogen 0.625 mg). Results are summarized in Fig. 3B and 4B. There appeared to be a decrease in the percentage of parabasal cells with bazedoxifene plus conjugated estrogen and ospemifene at all doses compared to placebo (not statistically significant). Bazedoxifene alone had no effect.
Network meta-analysis combining direct and indirect estimates demonstrated that soy isoflavone vaginal gel had the highest probability (34%) of being ranked as most effective drug and it produced the greatest change in vaginal pH (mean [95% CrI], −1.61 [6.26, 3.08]). Results are summarized in Fig. 3C and 4C. Ospemifene 60 mg/day and estriol vaginal gel showed the second highest effectiveness for vaginal pH (mean [95% CrI], −1.04 [−4.24, 2.20] for ospemifene 60 mg; mean [95% CrI], −1.04 [−6.50, 4.34] for estriol vaginal gel). Compared to placebo, sea buckthorn oil showed no significant effect on vaginal pH.
Network meta-analysis combining direct and indirect estimates demonstrated that ospemifene 60 mg/day had the highest probability (31%) of being ranked as most effective and was associated with the greatest increase in the improvement of dyspareunia (mean [95% CrI], 1.25 [−3.37, 5.88]). Ospemifene 30 mg/day also showed the identical ranked probability (31%) compared with ospemifene 60 mg/day, but had a smaller effect size with a wider 95% CrI (mean [95% CrI], 1.09 [−4.82, 7.07]). However, the interventions included in the meta-analysis exhibited almost no difference in effectiveness compared to placebo in improvement of dyspareunia. Results are summarized in Fig. 3D and 4D./
Network meta-analysis combining direct and indirect estimates demonstrated that ospemifene 30 mg/day had the highest probability (36%) of being ranked as most effective and was associated with the greatest improvement of vaginal dryness (mean [95% CrI], 0.89 [−3.10, 4.80]). Ospemifene 60 mg/day showed about the same effectiveness as ospemifene 30 mg/day (mean [95% CrI], 0.86 [−3.02, 4.72]), but the probability of its being ranked highest was less (27%). Results are summarized in Fig. 3E and 4E. The intervention with sea buckthorn oil was a failure compared with placebo in terms of the improvement of vaginal dryness.
Network meta-analysis combining direct and indirect estimates demonstrated that ospemifene 30 mg/day had the highest probability (36%) of being ranked first, and ospemifene 60 mg/day was associated with the greatest increase in endometrial thickness (mean [95% CrI], 0.64 [−3.12, 4.39]). Results are summarized in Fig. 3F and 4F. The two doses of ospemifene showed different results in effectiveness and ranked probability. This pattern seemed to result from the small effect size combined with wide CrIs. Although the differences were not significant, soy isoflavone vaginal gel and ospemifene 30 mg/day and 60 mg/day had greater effectiveness on endometrial thickness than did the placebo.
This systematic review has clearly described the results of nine 12-week clinical RCTs of AV treatment drugs. It is the first study to survey the comparative treatment effects of AV therapeutic agents for six vaginal symptoms using a network meta-analysis. Several drugs were indirectly compared with the placebo: estrogen, which is the typical AV therapeutic agent, bazedoxifene, estriol vaginal gel, ospemifene, sea buckthorn oil, and soy isoflavone vaginal gel. This indirect comparison identified reliable homogeneity of the clinical studies included in the analysis. All of the trials included in the analysis used an RCT design, and the patients were generally similar.
a common control, placebo, and the effects of other drugs, ospemifene 60 mg/day was shown to have the best outcomes in terms of superficial cells, parabasal cells, dyspareunia, and endometrial thickness. The effectiveness of ospemifene 30 mg/day was highest for vaginal dryness, but the effect size was not significantly different from that of ospemifene 60 mg/day. The effective probability and effective value of soy isoflavone vaginal gel were highest for vaginal pH. Generally, considering the improvement of AV symptoms, ospemifene was found to have the greatest therapeutic value. The high therapeutic effect of ospemifene with regard to the percentage of superficial cells and parabasal cells, dyspareunia, endometrial thickness, and vaginal dryness symptoms was correspond with the previous studies results, but the effect on vaginal pH was not consistent with previous findings.115
This systematic review with network meta-analysis has several strengths, including a narrowly focused literature search, attempts to maximize data outcomes through matching the units of variables, triplicate review procedures of the literature by specialists in obstetrics and gynecology and statistics, revision of effect estimates for inaccurate data through CrIs and SUCRA probabilities, and concurrent consideration of all variables by network meta-analysis. However, this review did not include many studies, and the duration of the included studies was only 12 weeks, which is short-term treatment. There is a need to evaluate long-term treatment studies to compare the efficacy of various drugs and to evaluate risk assessment bias.
This study compared the effects of several therapeutic agents on symptoms of AV through a network meta-analysis based on well-designed randomized controlled intervention studies. Ospemifene had greatest therapeutic effect on the percentage of superficial cells and parabasal cells and on symptoms of dyspareunia, vaginal dryness, and endometrial thickness; thus, ospemifene was confirmed to be effective in symptomatic AV therapy. This finding provides objective evidence for treatment and efficacy management of AV.
Figures and Tables
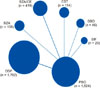 | Fig. 2The size of the nodes corresponds to the number of trials that study the treatments. Directly comparable treatments are linked with a line, the thickness of which corresponds to the number of trials that assess the comparison. PBO: placebo, BZA: bazedoxifene, CE: conjugated estrogens, EST: estriol vaginal gel, OSP: ospemifene, SBO: sea buckthorn oil, SIF: soy isoflavone vaginal gel. |
 | Fig. 3Forest plots for effect sizes of treatments for the outcomes. BZA: bazedoxifene, CrI: credible intervals. |
References
1. Nappi RE, Panay N, Bruyniks N, Castelo Branco C, De Villiers TJ, Simon JA. The clinical relevance of the effect of ospemifene on symptoms of vulvar and vaginal atrophy. Climacteric. 2015; 18:233–240.

2. Cui Y, Zong H, Yan H, Li N, Zhang Y. The efficacy and safety of ospemifene in treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy: a systematic review and meta-analysis. J Sex Med. 2014; 11:487–497.

3. Goldstein I, Dicks B, Kim NN, Hartzell R. Multidisciplinary overview of vaginal atrophy and associated genitourinary symptoms in postmenopausal women. Sex Med. 2013; 1:44–53.

4. Kim HK, Kang SY, Chung YJ, Kim JH, Kim MR. The recent review of the genitourinary syndrome of menopause. J Menopausal Med. 2015; 21:65–71.

5. Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2016; Cd001500.

6. Lee HH, Kim TH, Park J, Lee A, Park Y, Byun DW, et al. Expression of ezrin in vagina cells of postmenopausal rats after dietary administration of omega-3 Fatty Acid formula. J Menopausal Med. 2014; 20:97–103.

7. Santen RJ, Pinkerton JV, Conaway M, Ropka M, Wisniewski L, Demers L, et al. Treatment of urogenital atrophy with low-dose estradiol: preliminary results. Menopause. 2002; 9:179–187.

8. Wills S, Ravipati A, Venuturumilli P, Kresge C, Folkerd E, Dowsett M, et al. Effects of vaginal estrogens on serum estradiol levels in postmenopausal breast cancer survivors and women at risk of breast cancer taking an aromatase inhibitor or a selective estrogen receptor modulator. J Oncol Pract. 2012; 8:144–148.

9. Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause. 2010; 17:281–289.

10. Shin JJ, Kim SK, Lee JR, Suh CS. Ospemifene: A novel option for the treatment of vulvovaginal atrophy. J Menopausal Med. 2017; 23:79–84.

11. Jaisamrarn U, Triratanachat S, Chaikittisilpa S, Grob P, Prasauskas V, Taechakraichana N. Ultra-low-dose estriol and lactobacilli in the local treatment of postmenopausal vaginal atrophy. Climacteric. 2013; 16:347–355.

12. Lima SM, Bernardo BF, Yamada SS, Reis BF, da Silva GM, Galvão MA. Effects of Glycine max (L.) Merr. soy isoflavone vaginal gel on epithelium morphology and estrogen receptor expression in postmenopausal women: a 12-week, randomized, double-blind, placebo-controlled trial. Maturitas. 2014; 78:205–211.

13. Larmo PS, Yang B, Hyssala J, Kallio HP, Erkkola R. Effects of sea buckthorn oil intake on vaginal atrophy in postmenopausal women: a randomized, double-blind, placebo-controlled study. Maturitas. 2014; 79:316–321.

14. Kim SH, Park ES, Kim TH. Rejuvenation using plateletrich plasma and lipofilling for vaginal atrophy and lichen sclerosus. J Menopausal Med. 2017; 23:63–68.

15. DeGregorio MW, Zerbe RL, Wurz GT. Ospemifene: a first-in-class, non-hormonal selective estrogen receptor modulator approved for the treatment of dyspareunia associated with vulvar and vaginal atrophy. Steroids. 2014; 90:82–93.

16. Constantine G, Graham S, Portman DJ, Rosen RC, Kingsberg SA. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015; 18:226–232.

17. Cano A, Estèvez J, Usandizaga R, Gallo JL, Guinot M, Delgado JL, et al. The therapeutic effect of a new ultra low concentration estriol gel formulation (0.005% estriol vaginal gel) on symptoms and signs of postmenopausal vaginal atrophy: results from a pivotal phase III study. Menopause. 2012; 19:1130–1139.
18. Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010; 17:480–486.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download