Abstract
Objectives
Neopterin is a valuable diagnostic biomarker, which is elevated in inflammatory conditions like periodontitis, that is characterized by destruction of the supporting structures of the teeth. Among the biomarkers, neopterin occurs in body fluids, and acts as a diagnostic marker for present and future disease activity.
Methods
Thirty female subjects with chronic periodontitis, mean age 50 years (40-60 years) were included in this study. Depending upon their menstrual history, subjects were categorized into two groups of fifteen each. Group I 15 pre-menopausal women, and Group II 15 post-menopausal women. Saliva was collected, and neopterin levels were assessed by enzyme-linked immunosorbent assay in both the groups, at base line and after three months of nonsurgical periodontal therapy (NSPT). Periodontal parameters like pocket probing depth (PD) and Russell's periodontal disease index (PDI) were assessed before treatment as well as after three months of scaling and root planning.
Results
Intra group analysis showed significant markdown in the mean values of all the parameters from baseline to three months (P < 0.001), for all patients. The intergroup comparison, from baseline to 3 months also showed no significant change in PD and PDI values, but there was a statistically significant difference in the salivary neopterin levels (P = 0.04).
Periodontitis is an inflammatory disease that destroys both soft and hard tissues around the tooth. Recent trends in diagnostic research, includes measuring biomarkers, whereby prior risk to periodontitis will be identified. Circulating elevated levels of specific inflammatory markers can be correlated with the severity of the periodontal disease activity.1
The diagnosis of progressive phases of periodontal disease and classifying of patients who are at risk represents a major challenge for both investigators and clinicians. This identification can be accomplished by using biomarkers that are expressed in body fluids like gingival crevicular fluid (GCF), saliva and serum.2
A biomarker is the biologic measure that can be used as an index. It may be computed and evaluated as an indicator of normal, pathogenic biological processes or pharmacological responses to a therapeutic intervention.3 A traditional biomarker for periodontal disease is bleeding on probing (BOP), and is a reliable predictor available today. Researchers report that there are many false positive assumptions associated with it, but the absence of BOP is considered a very precise negative predictor of disease activity.3
Despite chronic periodontitis (CP) being a site specific disease, its susceptibility to a patient and site level is still unclear. Thereby, wide research activities were commenced since 1990 to evaluate the importance and correlation of individual biomarkers of periodontal disease activities, measured within GCF.4
Neopterin is an early and a valuable biomarker of cellular immunity, shown to be a sensitive assessment parameter for cell-mediated immune reactions. Hence determination of neopterin concentrations in distinctive body fluids is of diagnostic interest in a wide range of T lymphocytes and macrophages originated diseases. Increased neopterin production is also found in infections due to intracellular living bacteria and parasites.56
Neopterin concentration is directly proportional to the reactive oxygen species levels and is inversely related to the serum concentration of antioxidants like alpha-tocopherol. Hence it can be regarded as a marker of reactive oxygen species formed by the activated cellular immune system.7
The present study was undertaken to determine salivary neopterin levels in patients with periodontitis in pre- and post-menopausal women. We also aimed to evaluate the usefulness of neopterin as a biomarker to correlate the intensity of periodontal tissue destruction and its reverting response to nonsurgical periodontal therapy (NSPT).
The research decorum was approved by the Institutional Review Board (IRB) Committee at the Panineeya institute of dental sciences and research center. This study was registered under ClinicalTrials.gov identifier no. NCT02357745. Thirty female subjects with a mean age of 50 years (40-60 years) were included in this study. Subjects were sequentially enrolled, who met the selection criteria from January 2015 to August 2015. Study design was explained and an informed consent (written) was obtained prior to the study. Contingent upon menstrual history, subjects were categorized into two groups. Each group comprised of fifteen subjects. Group I and Group II encompass fifteen pre- and post-menopausal women with CP. All the individuals were diagnosed with CP which was conclusively proved by American Academy of Periodontology (AAP) classification.8 Periodontal parameters like pocket probing depth (PD) and Russell's periodontal disease index (PDI)9 were kept in view for this study. The study design included PD ≥ 4 mm and systemically healthy subjects from past six months with at least fifteen natural teeth, nonsmokers and persons who were neither on any medications nor undergone any dental procedures for the past six months were included in this study. Anyone on hormonal therapy, having other infections or pathology in oral cavity other than periodontitis were strictly counted out from this study. Saliva was collected and analyzed for neopterin in both the groups at baseline and three months after NSPT. Both PD and PDI were also measured with graduated William's periodontal probe (Hu-friedy, Chicago, IL, USA) before treatment as well as after three months of scaling and root planning (SRP).
Group I: Pre-menopause - Only age matched participants, who have menstrual irregularities caused due to age induced hormonal imbalance were included. Other menstrual disturbances were excluded. This was confirmed by gynecologist after thorough clinical and diagnostic evaluation.
Group II: Post-menopause - Age matched participants who were having amenorrhea for past 12 months or more with any obvious pathologic cause were substantiated by gynecologist.
The whole 1.5 milliliter of un-stimulated saliva was collected in a sterile plastic disposable cup (100 mL, PP; Sarstedt, Nümbrecht, Germany) by passive drool method. Patients were instructed not to swallow but to pool saliva until the desired quantity is obtained and were asked to tilt their heads, allowing the saliva to drool passively. Patients were advised not to brush or eat for 8 hours before collection of the sample. Use of mouthwash was strictly prohibited on the day of sample collection. Dental examination was not carried out 48 hours prior to saliva collection. Patients were requested to swill with normal water before sample collection. Samples were centrifuged (R-4C; REMI Laboratory Instruments, Mumbai, India) for 10 minutes at 15,000 g at 4℃ to remove any particulate matter.10 As per instructions of the manufacturer; samples were stored at -20℃ till six months for evaluation of neopterin. Complete procedure was carried out by single clinician under strict aseptic conditions.
Neopterin was determined by a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Neopterin kit; Alpha Diagnostic International, San Antonio, TX, USA). The basic concept of neopterin Elisa kit is specific competitive binding of human neopterin and enzyme-labeled neopterin with neopterin specific antibodies, immobilized on Microtiter plates. It was washed and change in colour was observed after addition of chromogenic substrate. Depending upon the amount of neopterin present, enzymatic reaction (blue color) was observed which is inversely proportional to neopterin in the sample. The reaction terminated after addition of stopping solution which changed the color from blue to yellow. Absorbance is then measured on an ELISA reader at 450 nm and the concentration of neopterin in samples and control is read from the standard curve (SC).
By determining the resulting concentration of the mean optical density (OD) of Calibrator A (based on 10 replicate analyses) minus 2 standard deviation (SD), lower detection limit is calculated from the SC.
Therefore, the sensitivity of the neopterin ELISA kit is 0.7 nmol/L.
The cross-reactivity was tested for these compounds with the direct neopterin ELISA kit with neopterin cross-reacting at 100%. No significant interference was detected at the following concentration: hemoglobin 35 mg/dL, bilirubin 2.25 mg/dL, triglyceride 125 mg/dL
According to the manufacturer's instructions the OD was measured at 450 nm by using ELISA 96-well micro plate reader. The amount of complexes bound to the plate and the OD is inversely proportional to the analytical concentration of the sample. All samples were measured in duplicates.
A. Average of the observance of all duplicates. Subtract the averaged non-specific binding (NSB) absorbance from the average obtained above. This yields the net absorbance. Divide the net absorbance by the net zero standard absorbance (Bo) to obtain the percent bound (B/Bo [%])
B. Formula
Abs.: average absorbance of duplicate wells, NSB: non-specific binding (also referred as the blank), Sample: particular serum or standard being calculated, Zero Standard: 0 nmol/L standard or 100% binding wells
C. plot a graph with percent bound on (Y-axis) and the concentration of the neopterin standards on (X-axis) starting with the 0.5 nmol/L point. Either logit-log or semi-log graph paper may be used. This yeilds the SC.
D. Using the SC, the neopterin concentration of each sample is determined.
Values that bind either higher or lower than the SC should not be determined by extrapolation.
The observations recorded were subjected to statistical analysis using IBM SPSS version 21 software (SPSS Inc., Chicago, IL, USA). The average of the clinical variables (PD, PDI for both the groups), the amount of saliva, and the amount of neopterin were calculated for each subject in both the groups and this mean value is used as the response variable. Results were symbolized as mean ± SD. The differences in clinical variables and amount of neopterin were examined using the Independent sample t test .The correlation between the levels of neopterin and clinical variables of the two groups were analyzed separately using paired t-test. P value < 0.05 was considered statistically significant.
Intra group comparison showed statistically significant reduction in the mean values of all the parameters from baseline to three months within the group I and II (Table 1).
In group I, the mean salivary neopterin levels were reduced from 13.63 ± 1.55 at baseline to 6.69 ± 1.24 in 3 months after treatment. The mean periodontal index levels have reduced from 6.08 ± 0.47 at baseline to 2.54 ± 0.12 in 3 months after treatment. In group II, the mean salivary neopterin levels were reduced from 15.61 ± 1.83 at baseline to 7.05 ± 1.41 in 3 months after treatment and mean periodontal index levels have reduced from 5.68 ± 0.65 at baseline to 2.53 ± 0.13 in 3 months after treatment. There was a statistically significant discrepancy seen from base line in 3 months of post treatment for all the parameters (P < 0.001) (Table 1, Fig. 1).
In the intergroup comparison, the mean concentration of neopterin levels in saliva at baseline was 13.63 ± 1.55 in group I and 15.61 ± 1.83 in group II. The mean values of periodontal index, at baseline was 6.08 ± 0.47 in group I and 5.68 ± 0.65 in group II. Comparing these values showed that the difference in the levels of neopterin between groups were statistically significant (P = 0.003) in saliva but not significant for periodontal index. After 3 months of post treatment, the periodontal index values were statistically insignificant in the inter-group comparison (Table 2, Fig. 2).
Neopterin is a biologically stable cellular immune activation marker which is easily looked over in human body fluids like GCF,11 the cubic measure of which optimizes with intensity of gingival inflammation. Consequentially this is useful for predicting the prognosis and diagnosis of severe form of periodontal diseases.12 Neopterin was first diagnosed in larvae of bees, in worker bees and in royal jelly in 1963, and subsequently in human urine by Sakurai and Goto13 in 1967.14 Neopterin is a metabolite of guanosine triphosphate (GTP), and in vitro interferon gamma (IFN-γ) is the only cytokine that induces the production of large amounts of neopterin in cell culture supernatants. On the other hand in human monocytes/macrophages and in some cell lines stimulated with this cytokine, neopterin is produced and released instead of other pteridine derivatives.1516 Periodontitis is a very common chronic infection or inflammation of the tissue surrounding the teeth. Severe attachment loss and bone loss progresses if untreated. However the nature and source of inflammation is unclear, but many studies have proven that periodontal therapy resolves the periodontal as well as systemic inflammation.171819 Recent studies demonstrated that, traditional clinical measures of human periodontal diseases are not designating of ongoing the future disease progression.20 Determination of the presence of inflammatory products found in GCF or saliva may be of value in evaluating both periodontal disease status and the outcome of therapy.21 Evaluation of neopterin status in various human biological fluids was done by many authors.222324 Exclusive studies on saliva, GCF as a biomarker of neopterin was also done by many authors.2324
Menopause accomplishes a wide range of changes in women's body, and the oral cavity is also not exceptional. As exalted levels of ovarian hormones, detected in pregnancy and oral contraceptive usage, can lead to an increase of gingival inflammation. On contrary, menopause shows depleted levels of ovarian sex steroids, which also causes worsening of gingival health.25 As far as gingiva is concerned, the effect of sex hormones is that they can influence the cellular proliferation, differentiation and ontogenesis of keratinocytes and fibroblasts. Estrogen is mainly responsible for alterations in the blood vessels and progesterone initiates the authoring of inflammatory mediators. Apart from these, estrogens can influence the cytodifferentiation of stratified squamous epithelium, synthesis and maintenance of fibrous collagen. Additionally, estrogen receptors (ERs) in cells like osteoblast contribute to direct mechanism of action on bone while ERs in periosteal fibroblasts and periodontal ligament fibroblasts implement a contrivance for direct action on different periodontal tissues.26 Both conditions have some common risk factors and they begin to show their effects mainly after the age of thirty five. The extent of the relationship between these two still remains uncertain.25 Estrogen has a number of effects on the modulation of the inflammatory response and immune cell function. These effects are largely mediated through ERs which are pre-eminently present on monocytes and macrophages.
Estrogen may induce an overall inhibitory effect on inflammation by suppressing pro inflammatory pathways. Bouman et al.27 and Bolego et al.28 found an enhanced inflammatory potential when macrophages individuate from the peripheral blood of the post-menopausal women were compared to those women of fertile age. Signaling reciprocal action is responsible by estrogen for the onset of the resolution phase and is able to shorten the perpetuation of the pro-inflammatory contingency and to direct the resolution of the inflammation towards the acquired deactivation stage. These findings may be conformant particularly for the identification of novel ER modulators efficacious in the prevention or mitigation of chronic inflammation.29 Reinforcing this affirmation many authors proved that alternative immune action of macrophages is reduced by estrogen treatment in menopause.30
Although few studies about neopterin activity in saliva were discussed,2223 but possible association with menopause and periodontitis salivary neopterin was not discussed earlier. As per our knowledge, this is the first study which correlated these three inflammatory conditions. In the present study, it was determined that the mean total amount of neopterin values of saliva was higher in both the groups, compared to normal values but significantly higher in the post- menopause group. There was reduction of mean values for periodontal treatment, but more significant reduction was seen in pre-menopause group. According to Novella et al.31 Post-menopausal women may have augmented circulating markers like TNF-α, which increases inflammation and increasing artery responsiveness to stimulate vasoconstriction. Studies demonstrated that NSPT reduces inflammation and improves periodontal status.32 Therefore it can be assumed that overall inflammatory markers also reduce after NSPT.171819 Ozmeric et al.33 has done a comparative study on neopterin levels in saliva, GCF and urine with aggressive periodontitis (AgP) patients, they suggested that mean neopterin levels were higher in saliva and GCF in AgP patients compared to controls. In our study also salivary neopterin levels were increased in both the groups and also decreased levels were seen in both the groups after NSPT, but significant reduction was seen in the pre-menopause group. This study result of reduced neopterin levels after NSPT are consistent with the study by Pradeep et al.34. The neopterin levels were correlated with the intensity of disease. The increase in neopterin with periodontal disease progression and decrease after treatment showed that neopterin is involved in the periodontal disease process, as confirmed by the previous study33 as well as the inflammatory conditions like menopause. Higher neopterin levels in a periodontal patient might reflect the enhanced macrophage infiltration to the periodontal lesion because neopterin is a macrophage activation marker.35 Macrophage collagenase may have a significant role in collagen destruction in diseased periodontal tissue. Therefore, we should consider changes in neopterin levels as an omen of the host mechanism leading to tissue destruction.33 Neopterin production was delineated by activated macrophages, this might be legitimate since macrophage infiltration and activation are known to be characteristics of chronic inflammation such as periodontitis.36 Neopterin is known to take part in nitric oxide syntheses (NOS) production to produce nitric oxide (NO).37 The presence of NO in saliva is mostly due to the salivary glands which may release NO and it mainly provides the natural antibacterial properties of saliva since it is powerful antimicrobial substance produced in response to periodontopathogens and local inflammatory changes.36 NO in saliva may be part of the non-specific natural defense mechanism of the oral cavity against pathogenic bacteria.38 Toniolo et al.30 stated that menopause is associated with macrophage activation profiles skewed towards pro-inflammatory phenotype. Macrophages, which play a direct and important function in cell mediated immunity, contain peroxidase, several acid hydrolases and collagenases.39 Macrophage collagenase may have a significant role in collagen destruction in diseased tissues of periodontium. Altered neopterin concentrations might be the beacon of these host mechanisms leading to tissue destruction. The results of the present study could be interpreted that higher salivary neopterin levels was seen in both the groups. But significantly higher in post-menopause with periodontitis might reflect the enhanced macrophage infiltration in menopause as well as periodontal lesion since neopterin is also a macrophage activation marker. Determination of treatment should be beneficial in observing the direct relationship between neopterin and periodontal status. The decrease in the salivary neopterin levels after the treatment seems to indicate the effectiveness of the treatment. Within the limits of the study the difference at baseline and at follow up therapy indicated not only a significant reduction in the neopterin levels, but also an improvement in the clinical parameters of the patients with periodontal disease and there was visible gingival tissue improvement. With this study, it can be put forward that NSPT is an adjunctive therapy to control inflammation. However, salivary neopterin levels did not seem to be a precise marker of clinical status. To confirm our presupposition, longitudinal studies conducted on larger patient populations are mandatory.
1. As post-menopause being natural, hysterectomy was not categorized.
2. Early menopause and normal menopause stage were not categorized.
3. Detection of follicle stimulating hormone (FSH) fluctuations in the blood sample is the bench mark to identify pre- or post-menopause, which was not done.
Our data indicated that the immune activation biomarker neopterin might be closely associated with the initiation and progression of periodontal disease as well as in menopause and thereby it further contributes to collagen degradation as well as being an indicator of the cogency of the periodontal treatment. Nevertheless, more clinical investigations conducted on larger scale are conspicuously necessitated for ancillary firmness of the neopterin levels in oral biological fluids in such conditions. Advancements in the research field at cellular level are required for the early detection of these complexities, thus enabling us to intervene at a very early stage.
Determination of neopterin levels before and after periodontal treatment should be beneficial in observing the direct relationship between neopterin levels and periodontal status. In future studies, differentiating recent menopause and prolonged menopause stages might give more accurate results, because more hazardous effects might be observed in prolonged menopausal stage. Accurate detection of intracellular action and function of macrophages may give exact results. To conclude, long-term and well controlled clinical trials in a large population are to be carried out for proper authentication. Thus specific salivary markers significant for diagnosis and monitoring of periodontitis are to be determined at molecular levels.
Figures and Tables
Fig. 1
(A) Intra group comparison of salivary neopterin. (B) Intra group comparison of periodontal index.

Fig. 2
(A) Intra group comparison of salivary neopterin. (B) Intra group comparison of periodontal index.

References
1. de Queiroz AC, Taba M Jr, O'Connell PA, da Nobrega PB, Costa PP, Kawata VK, et al. Inflammation markers in healthy and periodontitis patients: a preliminary data screening. Braz Dent J. 2008; 19:3–8.
2. Ebersole JL, Machen RL, Steffen MJ, Willmann DE. Systemic acute-phase reactants, C-reactive protein and haptoglobin, in adult periodontitis. Clin Exp Immunol. 1997; 107:347–352.
3. Zhang L, Henson BS, Camargo PM, Wong DT. The clinical value of salivary biomarkers for periodontal disease. Periodontol 2000. 2009; 51:25–37.
4. Chapple IL. Periodontal diagnosis and treatment--where does the future lie? Periodontol 2000. 2009; 51:9–24.
5. Marsálek P, Svoboda M, Smutná M, Blahová J, Vecerek V. Neopterin and biopterin as biomarkers of immune system activation associated with castration in piglets. J Anim Sci. 2011; 89:1758–1762.
6. Andert SE, Müller MM. Neopterin: biochemistry and clinical usefulness. J Int Fed Clin Chem. 1995; 7:70–76.
7. Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab. 2002; 3:175–187.
8. Armitage GC. Development of a classification system for periodontal diseases and conditions. Northwest Dent. 2000; 79:31–35.
9. Wei SH, Lang KP. Periodontal epidemiological indices for children and adolescents: I. Gingival and periodontal health assessments. Pediatr Dent. 1981; 3:353–360.
10. Elgün S, Ozmeriç N, Demirtaş S. Alanine aminopeptidase and dipeptidylpeptidase IV in saliva: the possible role in periodontal disease. Clin Chim Acta. 2000; 298:187–191.
11. Eisenhut M. Neopterin in diagnosis and monitoring of infectious diseases. J Biomark. 2013; 2013:196432.
12. Bodur A, Baydar T, Ozmeric N, Engin AB, Uraz A, Eren K, et al. Neopterin profile to evaluate the effectiveness of treatment in aggressive periodontitis. Pteridines. 2003; 14:77–81.
13. Sakurai A, Goto M. Neopterin: isolation from human urine. J Biochem. 1967; 61:142–145.
14. Fuchs D, Weiss G, Wachter H. Neopterin, biochemistry and clinical use as a marker for cellular immune reactions. Int Arch Allergy Immunol. 1993; 101:1–6.
15. Huber C, Batchelor JR, Fuchs D, Hausen A, Lang A, Niederwieser D, et al. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med. 1984; 160:310–316.
16. Ayling J, Nair MG, Baugh CM, editors. Chemistry and biology of pteridines and folates. New York, NY: Springer Science+Business Media New York;1993.
17. George AK, Janam P. The short-term effects of non-surgical periodontal therapy on the circulating levels of interleukin-6 and C-reactive protein in patients with chronic periodontitis. J Indian Soc Periodontol. 2013; 17:36–41.
18. Li C, Lv Z, Shi Z, Zhu Y, Wu Y, Li L. Periodontal therapy for the management of cardiovascular disease in patients with chronic periodontitis. Cochrane Database Syst Rev. 2014; Cd009197.
19. D'Aiuto F, Nibali L, Parkar M, Suvan J, Tonetti MS. Short-term effects of intensive periodontal therapy on serum inflammatory markers and cholesterol. J Dent Res. 2005; 84:269–273.
20. Bolerázska B, Mareková M, Markovská N. Trends in laboratory diagnostic methods in periodontology. Acta Medica (Hradec Kralove). 2016; 59:3–9.
21. Ram VS, Parthiban , Sudhakar U, Mithradas N, Prabhakar R. Bonebiomarkers in periodontal disease: a review article. J Clin Diagn Res. 2015; 9:Ze07–Ze10.
22. Salazar MG, Jehmlich N, Murr A, Dhople VM, Holtfreter B, Hammer E, et al. Identification of periodontitis associated changes in the proteome of whole human saliva by mass spectrometric analysis. J Clin Periodontol. 2013; 40:825–832.
23. Mahendra L, Mahendra J, Borra SK, Nagarajan A. Estimation of salivary neopterin in chronic periodontitis. Indian J Dent Res. 2014; 25:794–796.
24. Arjunkumar R, Sudhakar U, Jayakumar P, Arunachalam L, Suresh S, Virupapuram P. Comparative analysis of gingival crevicular fluid neopterin levels in health and periodontal disease: a biochemical study. Indian J Dent Res. 2013; 24:582–586.
25. Suri V, Suri V. Menopause and oral health. J Midlife Health. 2014; 5:115–120.
26. Dutt P, Chaudhary S, Kumar P. Oral health and menopause: a comprehensive review on current knowledge and associated dental management. Ann Med Health Sci Res. 2013; 3:320–323.
27. Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005; 11:411–423.
28. Bolego C, Cignarella A, Staels B, Chinetti-Gbaguidi G. Macrophage function and polarization in cardiovascular disease: a role of estrogen signaling? Arterioscler Thromb Vasc Biol. 2013; 33:1127–1134.
29. Villa A, Rizzi N, Vegeto E, Ciana P, Maggi A. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci Rep. 2015; 5:15224.
30. Toniolo A, Fadini GP, Tedesco S, Cappellari R, Vegeto E, Maggi A, et al. Alternative activation of human macrophages is rescued by estrogen treatment in vitro and impaired by menopausal status. J Clin Endocrinol Metab. 2015; 100:E50–E58.
31. Novella S, Heras M, Hermenegildo C, Dantas AP. Effects of estrogen on vascular inflammation: a matter of timing. Arterioscler Thromb Vasc Biol. 2012; 32:2035–2042.
32. Acharya AB, Thakur S, Muddapur MV. Effect of scaling and root planing on serum interleukin-10 levels and glycemic control in chronic periodontitis and type 2 diabetes mellitus. J Indian Soc Periodontol. 2015; 19:188–193.
33. Ozmeric N, Baydar T, Bodur A, Engin AB, Uraz A, Eren K, et al. Level of neopterin, a marker of immune cell activation in gingival crevicular fluid, saliva, and urine in patients with aggressive periodontitis. J Periodontol. 2002; 73:720–725.
34. Pradeep AR, Kumar MS, Ramachandraprasad MV, Shikha C. Gingival crevicular fluid levels of neopterin in healthy subjects and in patients with different periodontal diseases. J Periodontol. 2007; 78:1962–1967.
35. Roitt IM, Brostoff J, Male DK. Immunology. 4th ed. London, UK: Mosby;1996.
36. Cortran RS, Kumar V, Robbins SL. Inflammation and repair. In : Cotran RS, Kumar V, Robbins SL, editors. Robbins' pathologic basis of disease. 5th ed. Philadelphia, PA: W.B. Saunders;1994. p. 51–92.
37. Schobersberger W, Hoffmann G, Grote J, Wachter H, Fuchs D. Induction of inducible nitric oxide synthase expression by neopterin in vascular smooth muscle cells. FEBS Lett. 1995; 377:461–464.
38. Flannagan RS, Heit B, Heinrichs DE. Antimicrobial mechanisms of macrophages and the immune evasion strategies of staphylococcus aureus. Pathogens. 2015; 4:826–868.
39. Hibbs JB Jr, Taintor RR, Vavrin Z, Rachlin EM. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988; 157:87–94.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


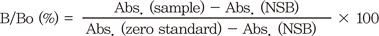




 XML Download
XML Download