Abstract
Objectives
Ovarian hormones have been shown to regulate body weight, intra-abdominal fat accumulation and plasma level of cytokines. The aim of this study was to investigate the effect of estrogen replacement therapy on visceral adipose tissue, plasma level of apelin, lipid profiles, and glucose in ovariectomized (OVX) rats.
Methods
Thirty female Wistar rats were divided into OVX (n = 20) and sham (n = 10) groups. OVX rats were subdivided into estrogen replacement therapy (OVX+est; n = 10) receiving 17 β-estradiol valerates (30 µg/kg, s.c., 5 day/week, for eight weeks), and vehicle control group receiving sesame oil same as experiment group (OVX+ses oil; n = 10). After the treatments, all groups were sacrificed and blood samples were collected, visceral fats were taken from the abdominal cavity and weighed immediately. Apelin were measured using enzyme-linked immunosorbent assay kits. Lipid profiles and glucose were measured using the enzymatic colorimetric method. Data were analyzed with one-way analysis of variance and (P < 0.05) determined as the statistical significance level.
Results
After eight weeks, body weight, body mass index (BMI), visceral fat, apelin and lipid profiles (P < 0.01) were increased significantly in OVX rats compared to sham group. Treatment with estrogen leads to significant reduction in body weight and BMI (P < 0.05), there was no significant change in serum apelin level in OVX+est rats compared to OVX+ses.
Obesity is now recognized as a major public health problem that constitutes a risk factor for life-threatening diseases such as type 2 diabetes and cardiovascular disease (CVD), as well as some types of cancer.1 These disorders represent major causes of morbidity and mortality in industrialized countries and are increasing problems in developing countries as well.2
Obesity as the most common disorders in climacteric women occurs in approximately 65% of them3 due to alterations in visceral adipose tissue metabolism.45 Menopause is associated with a rise in follicle-stimulating hormone and luteinizing hormone levels and a fall in estrogen.6789 Women have more body fat compared to men, and there is a gender-specific difference in fat distribution. In women, adipose tissue is accumulated especially around the hips, buttocks, and thighs while men have a larger intra-abdominal fat mass.1011 Pedersen et al.12 demonstrated that estradiol, binding to its receptor α, inhibits adrenaline-stimulated lipolysis in human subcutaneous fat cells by increasing the amount of α2-adrenergic anti-lipolytic receptors. This may explain how estradiol is related to typical female subcutaneous adipose tissue distribution because this inhibition is not observed in visceral fat depots. In women, estradiol may shift accumulation of fat from visceral into subcutaneous depots.12 Redistribution of body fat, after menopause, is associated with CVD1011 and metabolic syndrome as well.13
In a recent study, ovariectomy (OVX) in rats resulted in a progressive accumulation of fat in the abdomen, increased risks of insulin resistance, dyslipidemia and CVDs.1415 Adipokines are an exciting new link between obesity, insulin resistance and CVD.1516 Apelin is a recently identified bioactive adipokine. It is an endogenous of the orphan seven-transmembrane (TM) domain G protein-coupled receptor, putative receptor protein related to the angiotensin receptor AT1 (APJ), which is functionally similar to angiotensin-1 coupled receptor.1718
Existence of apelin and its receptor in various tissues of human and mice such as: hypothalamus, anterior pituitary, endothelial vessels, heart, lungs, stomach, kidney, mammary glands, thyroid, ovarian, colon mucus, pancreas, adipose tissue, liver and muscle reveal the widespread role of this hormone.181920212223242526272829 The hormone regulates adipose tissue's growth and has a role in the process of chronic inflammation caused by increased adipose tissue and stress.2428 Apelin upregulates sex steroids, insulin, tumor necrosis factor-α and growth hormone in adipose tissues of human and mice.1923242526272829 This cytokine is involved in regulation of fluids and food intake in the hypothalamus22 and increases in apelin concentration results in energy consumption.303132
Regarding the fact that visceral fat is the source of adipokines, more notably, apelin, we hypothesized that estrogen deficiency might be one possibility for apelin level reduction and consequently visceral fat accumulation with other clusters of metabolic syndrome components.
To our knowledge relationship between plasma apelin concentration and estrogen treatment has not been studied yet. Therefore, the purpose of this study was to determine the effect of estrogen replacement therapy on visceral fat, serum apelin level. To approach these goals, we used OVX rats as a model of menopause.3334353637
Female Wistar rats (n = 30), weighing 180 to 200 g (2 months of age) were used in this study. Except for sham group, all animal underwent OVX surgery according to the technique described by Babaei et al..33 For surgery, rats were anesthetized using a mixture of ketamine- xylazine (61.5-7.6 mg/kg, i.p.). Animals were housed four per cage and fed standard pellet rat chow and had free access to tap water. The 12:12-hour light: dark cycle started at 07:00 hour and the room temperature was kept at 21 to 23oC. The experiments described in this report were conducted according to the policy of Ethics Committee of the Guilan University of Medical Sciences.
Thirty Female Wistar rats were divided into three following groups: OVX (n = 20) and sham (n = 10) groups. OVX rats were subdivided into estrogen replacement therapy (OVX+est; n = 10), and sesame oil therapy (OVX+ses oil; n = 10) groups. Rats in the OVX+est group received subcutaneous injections of 17β-estradiol valerate (30 mg/kg body weight; Sigma-Aldrich Corp., St. Louis, MO, USA) dissolved in 0.2 mL sesame oil (Aburaihan Pharma Co., Tehran, Iran), three days a week, for eight weeks. Rats in the OVX+ses oil group received the same volume of sesame oil. All rats were weighed twice a week, between 09:00 and 11:00 to yield a weekly weight for each animal, two weights were averaged. To measure the food intake, an equal amount of food (20 g/day/rat) was given to all rats and their consumption was measured the following day by subtracting the remaining uneaten food from the total given one. All experimental groups had initially similar mean body mass and were treated similarly in terms of daily manipulations. Body weight, food intake, and visceral fat were measured using the accurate to 0.1 g scale (Sartorius, Goettingen, Germany).
All rats were weighed twice a week, between 09:00 and 11:00. A weight average was obtained for each animal. The height of the animal was measured under general anesthesia from tip of nose to the anus. BMI was measured dividing weight by the square of height (g/cm2).
The food was removed from the animal's cage at least 24 hours before scarification. After complete anesthesia, the abdominal cavity was rapidly opened and blood samples were drawn from the inferior vena cava. The serum was immediately separated by centrifugation (3,000 rpm for 15 minutes) and stored at -80℃ for later biochemical and hormonal measurements.
Serum apelin concentration was measured by rat apelin enzyme-linked immunosorbent assay kit (Shanghai Crystal day Biotech Co., Shanghai, China). The serum glucose concentration was determined by enzymatic (glucose oxidase-amino antipyrine) colorimetric method (Pars Azmoun, Tehran, Iran) and Serum total cholesterol (TC) and high-density lipoprotein cholesterol (HDL-C) were determined by enzymatic (Pars Azmoun) colorimetric method. Serum triglyceride (TG) was determined by enzymatic (Pars Azmoun) colorimetric method. Serum glucose, cholesterol, TG, HDLC, and low-density lipoproteins cholesterol I (LDL-C) were determined like our previous works.3839 After collecting the blood samples, all intra-abdominal fat depots including mesenteric, urogenital and retroperitoneal were dissected out by one experimenter and weighed immediately after dissection to avoid evaporative weight loss. Mesenteric fat pad consisted of adipose tissue surrounding the gastrointestinal tract from the gastroesophageal sphincter to the end of the rectum. Urogenital fat pad included adipose tissue surrounding the kidneys, ureters, and bladder as well as ovaries, oviducts, and uterus. The retroperitoneal fat pad was taken distinctly behind each kidney along the lumbar muscles.
All data are presented as mean ± standard error (SE) before statistical analysis, normal distribution and homogeneity of the variances were tested by K-S test. Statistical comparisons between groups were performed by one-way analysis of variance test, followed by Tukey's post-hoc test. Levels of statistical significance were set at P < 0.05.
Two weeks after OVX, visceral fat (P = 0.006) and body weight (P = 0.001) of OVX group were significantly increased compared with the sham group and remained elevated even after 8 weeks of surgery (P = 0.02, P = 0.004) respectively (Table 1).
Serum glucose, TC (P = 0.004) and HDL-C (P = 0.027) and LDL-C (P = 0.022) also showed elevation after two weeks of OVX.
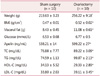
No significant difference was observed in visceral fat, blood lipids and plasma apelin concentration between OVX+est and OVX+ses oil groups after eight weeks of treatment. However, estrogen therapy reduced body weight compared with the OVX+ses oil group (Table 2).
Our results showed a significant difference in body weight, height, BMI, visceral fat, weight, glucose level, blood lipid profile, and serum apelin between the OVX and sham groups, two weeks after surgery. These findings partly are in agreement with the results of our previous study40 and Al-Wahaibi and colleagues41 indicating the significant increase in body weight of OVX rats after one month.
Ten weeks follow-up in our study showed that increase in body weight and visceral fat in OVX rats remained elevated compared to the control group. This is in agreement with the findings of several studies showed an increase in visceral fat after hypoestrogenism.414243 OVX may stimulate adipocyte hypertrophy and increased levels of epidermal growth factor, therefore OVX rats will be promoting obesity.3839
The results of 8-week estrogen therapy in the present study revealed that a significant reduction in body weight and BMI. While no significant difference was observed in visceral fat, blood lipids and plasma apelin concentration between OVX+est and OVX+ses oil groups after eight weeks of treatment.
Based on the results of studies in in-vivo and in-vitro condition, estrogen receptors play a major role in the regulation and distribution of body fat and are correspondence for lipolytic effects of estrogen.44 Therefore, our results in line with other similar studies42434546 indicate an increase in body weight after OVX with increase of fat mass, especially visceral one. No significant difference in the average of food intake between OVX+est and OVX+ses oil groups confirms that estrogen induces weight loss through other mechanisms rather than central mechanisms of food intake. Although the mechanisms of estrogen on body weight has not been fully understood, but the study carried out by Ryan et al.47 showed that ovarian steroid hormones involved in the regulation of obese gene and corticosterone hormone.
According to D'Eon et al.48 estrogen therapy leads to weight reduction. In the present study, a significant reduction in body weight after estrogen therapy is not in parallel with a reduction in visceral fat. The data found in our study on visceral fat is consistent with Ryan et al.47 and Sites et al.49 in postmenopausal women after hormone replacement therap. However, some studies showing contradictory results of estrogen on visceral fat in postmenopausal women495051 and OVX rats as well.42 D'Eon et al.48 were reported a reduction in visceral adipose tissue and adipocytes size in OVX rats after estrogen therapy. They found increased rate of adipocytes lipolysis and down-regulation of related genes, such as lipoprotein lipase in abdominal adipose tissue after injection of estradiol. They also showed that in muscles, estradiol led to up-regulation of lipoprotein lipase enzyme and peroxisome proliferator-activated receptor (PPAR-γ).48 Therefore, fat oxidation and adipocytes lipolysis cause fat usage as the main source of body fuel.42 In addition, the loss of ovarian estrogen causes increasing in cell size, lipolysis speed and lipoprotein lipase activity in visceral adipose tissue.4852 The findings of this study showed insignificant changes in serum apelin after eight-week in the OVX+est compare to OVX+ses oil group. While serum TG levels in response to estrogen therapy were increased insignificantly. This finding is consistent with results of the study showed that hormone therapy has a negative impact on TG levels.51 This finding is in contradictory with the results of those studies reported that hormone therapy has a positive effect on the cardiovascular system.5153 However, there is little information for explaining the results of the effects of estrogen therapy on the relationship between apelin and visceral fat level. Cross-sectional and short-term replacement of estradiol, the method for measuring visceral fat can likelihood affect the relationship between the two variables. Studies have shown that hypoestrogenism coincides with hyperlipidemia, hyperinsulinemia, and hypertension, the most important components of metabolic syndrome.54 On the other hand, increasing in cytokines such as apelin is associated with hypertension, heart failure, obesity,5556 hyperglycemia and atherogenic effects5758 and also according to studies Apelin can be used as a specific marker for insulin sensitivity and lipid profile and this adipokine might play a role in the pathogenesis of polycystic ovarian syndrome.596061626364 No significant change in serum apelin of OVX rats after estrogen replacement indicates that serum apelin concentration was found to be regulated by other factors such as nutrition, growth hormone, cortisol,29 and also liver function65 rather than estrogen per se. Future studies with long-term intervention are suggested to clarify the relationship between the cytokine apelin and estrogen considering molecular signaling.
Figures and Tables
Table 2
Metabolic, hormonal, and morphometric variables after eight-week estrogen therapy

*Ovariectomized rats reciving 17β-estradiol replacement
†Ovariectomized rats receiving sesame oil
‡P < 0.05 vs. OVX + oil group (n = 10)
BMI: body mass index, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, OVX: ovariectomy, Est: estradiol, Ses oil: sesame oil
References
1. Davidson LE, Hudson R, Kilpatrick K, Kuk JL, McMillan K, Janiszewski PM, et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med. 2009; 169:122–131.
2. Mattu HS, Randeva HS. Role of adipokines in cardiovascular disease. J Endocrinol. 2013; 216:T17–T36.
3. Paszkowski T, Kłodnicka M. Hormonalna terapia zastępcza. Przegl Menopauzalny. 2007; 2:106–109.
4. Ferrara CM, Lynch NA, Nicklas BJ, Ryan AS, Berman DM. Differences in adipose tissue metabolism between postmenopausal and perimenopausal women. J Clin Endocrinol Metab. 2002; 87:4166–4170.
5. Foster MT, Pagliassotti MJ. Metabolic alterations following visceral fat removal and expansion: Beyond anatomic location. Adipocyte. 2012; 1:192–199.
6. Augoulea A, Mastorakos G, Lambrinoudaki I, Christodoulakos G, Creatsas G. Role of postmenopausal hormone replacement therapy on body fat gain and leptin levels. Gynecol Endocrinol. 2005; 20:227–235.
7. Blake J. Menopause: evidence-based practice. Best Pract Res Clin Obstet Gynaecol. 2006; 20:799–839.
8. Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Recent Prog Horm Res. 2002; 57:257–275.
9. Utian WH. Menopause-related definitions. Int Congr Ser. 2004; 1266:133–138.
10. Mattsson C, Olsson T. Estrogens and glucocorticoid hormones in adipose tissue metabolism. Curr Med Chem. 2007; 14:2918–2924.
11. Misso ML, Jang C, Adams J, Tran J, Murata Y, Bell R, et al. Differential expression of factors involved in fat metabolism with age and the menopause transition. Maturitas. 2005; 51:299–306.
12. Pedersen SB, Kristensen K, Hermann PA, Katzenellenbogen JA, Richelsen B. Estrogen controls lipolysis by upregulating alpha2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor alpha. Implications for the female fat distribution. J Clin Endocrinol Metab. 2004; 89:1869–1878.
13. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002; 287:356–359.
14. Cekmez F, Canpolat FE, Pirgon O, Çetinkaya M, Aydinoz S, Suleymanoglu S, et al. Apelin, vaspin, visfatin and adiponectin in large for gestational age infants with insulin resistance. Cytokine. 2011; 56:387–391.
15. Corriveau P, Paquette A, Brochu M, Prud’homme D, Rabasa-Lhoret R, Lavoie JM. Resistance training prevents liver fat accumulation in ovariectomized rats. Maturitas. 2008; 59:259–267.
16. Babaei P, Damirchi A, Hoseini R. The interaction effects of aerobic exercise training and vitamin D supplementation on plasma lipid profiles and insulin resistance in ovariectomized rats. J Exerc Nutrition Biochem. 2015; 19:173–182.
17. Edinger AL, Hoffman TL, Sharron M, Lee B, Yi Y, Choe W, et al. An orphan seven-transmembrane domain receptor expressed widely in the brain functions as a coreceptor for human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol. 1998; 72:7934–7940.
18. Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998; 251:471–476.
19. Hosoya M, Kawamata Y, Fukusumi S, Fujii R, Habata Y, Hinuma S, et al. Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J Biol Chem. 2000; 275:21061–21067.
20. Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, et al. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000; 74:34–41.
21. O'Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, et al. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993; 136:355–360.
22. Kawamata Y, Habata Y, Fukusumi S, Hosoya M, Fujii R, Hinuma S, et al. Molecular properties of apelin: tissue distribution and receptor binding. Biochim Biophys Acta. 2001; 1538:162–171.
23. Devic E, Paquereau L, Vernier P, Knibiehler B, Audigier Y. Expression of a new G protein-coupled receptor X-msr is associated with an endothelial lineage in Xenopus laevis. Mech Dev. 1996; 59:129–140.
24. Esteve E, Ricart W, Fernández-Real JM. Adipocytokines and insulin resistance: the possible role of lipocalin-2, retinol binding protein-4, and adiponectin. Diabetes Care. 2009; 32:Suppl 2. S362–S367.
25. Hus-Citharel A, Bouby N, Frugière A, Bodineau L, Gasc JM, Llorens-Cortes C. Effect of apelin on glomerular hemodynamic function in the rat kidney. Kidney Int. 2008; 74:486–494.
26. Medhurst AD, Jennings CA, Robbins MJ, Davis RP, Ellis C, Winborn KY, et al. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J Neurochem. 2003; 84:1162–1172.
27. Tasci I, Dogru T, Naharci I, Erdem G, Yilmaz MI, Sonmez A, et al. Plasma apelin is lower in patients with elevated LDL-cholesterol. Exp Clin Endocrinol Diabetes. 2007; 115:428–432.
28. Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004; 92:347–355.
29. Wei L, Hou X, Tatemoto K. Regulation of apelin mRNA expression by insulin and glucocorticoids in mouse 3T3-L1 adipocytes. Regul Pept. 2005; 132:27–32.
30. Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC, et al. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003; 52:268–276.
31. Chapman NA, Dupre DJ, Rainey JK. The apelin receptor: physiology, pathology, cell signalling, and ligand modulation of a peptide-activated class A GPCR. Biochem Cell Biol. 2014; 92:431–440.
32. Higuchi K, Masaki T, Gotoh K, Chiba S, Katsuragi I, Tanaka K, et al. Apelin, an APJ receptor ligand, regulates body adiposity and favors the messenger ribonucleic acid expression of uncoupling proteins in mice. Endocrinology. 2007; 148:2690–2697.
33. Babaei P, Mehdizadeh R, Ansar MM, Damirchi A. Effects of ovariectomy and estrogen replacement therapy on visceral adipose tissue and serum adiponectin levels in rats. Menopause Int. 2010; 16:100–104.
34. Damirchi A, Mehdizade R, Ansar MM, Soltani B, Babaei P. Effects of aerobic exercise training on visceral fat and serum adiponectin concentration in ovariectomized rats. Climacteric. 2010; 13:171–178.
35. Saengsirisuwan V, Pongseeda S, Prasannarong M, Vichaiwong K, Toskulkao C. Modulation of insulin resistance in ovariectomized rats by endurance exercise training and estrogen replacement. Metabolism. 2009; 58:38–47.
36. Zoth N, Weigt C, Laudenbach-Leschowski U, Diel P. Physical activity and estrogen treatment reduce visceral body fat and serum levels of leptin in an additive manner in a diet induced animal model of obesity. J Steroid Biochem Mol Biol. 2010; 122:100–105.
37. Zoth N, Weigt C, Zengin S, Selder O, Selke N, Kalicinski M, et al. Metabolic effects of estrogen substitution in combination with targeted exercise training on the therapy of obesity in ovariectomized Wistar rats. J Steroid Biochem Mol Biol. 2012; 130:64–72.
38. Azman A, Khalid BAK, Ima-Nirwana S. The effects of vitamin E on bodyweight and fat mass in intact and ovariectomized female rats. Med J Islamic World Acad Sci. 2001; 14:125–138.
39. Wang JF, Guo YX, Niu JZ, Liu J, Wang LQ, Li PH. Effects of Radix Puerariae flavones on liver lipid metabolism in ovariectomized rats. World J Gastroenterol. 2004; 10:1967–1970.
40. Hoseini R, Damirchi A, Babaei P. Vitamin D increases PPARgamma expression and promotes beneficial effects of physical activity in metabolic syndrome. Nutrition. 2017; 36:54–59.
41. Al-Wahaibi A, Farihah HS, Azian AL, Wan Nazaimoon WM. Effects of ovariectomy on body weight and activity of 11-beta hydroxysteroid dehydrogenase type I In the liver and adipose tissue of rats. Sci J King Faisal Univ. 2008; 9:149–162.
42. Choi SB, Jang JS, Park S. Estrogen and exercise may enhance beta-cell function and mass via insulin receptor substrate 2 induction in ovariectomized diabetic rats. Endocrinology. 2005; 146:4786–4794.
43. Meli R, Pacilio M, Raso GM, Esposito E, Coppola A, Nasti A, et al. Estrogen and raloxifene modulate leptin and its receptor in hypothalamus and adipose tissue from ovariectomized rats. Endocrinology. 2004; 145:3115–3121.
44. Mueller SO, Korach KS. Immortalized testis cell lines from estrogen receptor (ER) alpha knock-out and wild-type mice expressing functional ERalpha or ERbeta. J Androl. 2001; 22:652–664.
45. Choi KM, Lee J, Lee KW, Seo JA, Oh JH, Kim SG, et al. The associations between plasma adiponectin, ghrelin levels and cardiovascular risk factors. Eur J Endocrinol. 2004; 150:715–718.
46. Latour MG, Shinoda M, Lavoie JM. Metabolic effects of physical training in ovariectomized and hyperestrogenic rats. J Appl Physiol (1985). 2001; 90:235–241.
47. Ryan AS, Nicklas BJ, Berman DM. Hormone replacement therapy, insulin sensitivity, and abdominal obesity in postmenopausal women. Diabetes Care. 2002; 25:127–133.
48. D'Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem. 2005; 280:35983–35991.
49. Sites CK, Brochu M, Tchernof A, Poehlman ET. Relationship between hormone replacement therapy use with body fat distribution and insulin sensitivity in obese postmenopausal women. Metabolism. 2001; 50:835–840.
50. Munoz J, Derstine A, Gower BA. Fat distribution and insulin sensitivity in postmenopausal women: influence of hormone replacement. Obes Res. 2002; 10:424–431.
51. Sumino H, Ichikawa S, Yoshida A, Murakami M, Kanda T, Mizunuma H, et al. Effects of hormone replacement therapy on weight, abdominal fat distribution, and lipid levels in Japanese postmenopausal women. Int J Obes Relat Metab Disord. 2003; 27:1044–1051.
52. Tchernof A, Desmeules A, Richard C, Laberge P, Daris M, Mailloux J, et al. Ovarian hormone status and abdominal visceral adipose tissue metabolism. J Clin Endocrinol Metab. 2004; 89:3425–3430.
53. Wakatsuki A, Ikenoue N, Okatani Y, Fukaya T. Estrogen-induced small low density lipoprotein particles may be atherogenic in postmenopausal women. J Am Coll Cardiol. 2001; 37:425–430.
54. Heinonen MV, Purhonen AK, Miettinen P, Pääkkönen M, Pirinen E, Alhava E, et al. Apelin, orexin-A and leptin plasma levels in morbid obesity and effect of gastric banding. Regul Pept. 2005; 130:7–13.
55. Boucher J, Masri B, Daviaud D, Gesta S, Guigné C, Mazzucotelli A, et al. Apelin, a newly identified adipokine upregulated by insulin and obesity. Endocrinology. 2005; 146:1764–1771.
56. Kleinz MJ, Davenport AP. Emerging roles of apelin in biology and medicine. Pharmacol Ther. 2005; 107:198–211.
57. Li L, Yang G, Li Q, Tang Y, Yang M, Yang H, et al. Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Exp Clin Endocrinol Diabetes. 2006; 114:544–548.
58. Rittig K, Hildebrandt U, Thamer C, Staiger H, Peter A, Stefan N, et al. Apelin serum levels are not associated with early atherosclerosis or fat distribution in young subjects with increased risk for type 2 diabetes. Exp Clin Endocrinol Diabetes. 2011; 119:358–361.
59. Altinkaya SÖ, Nergiz S, Küçük M, Yüksel H. Apelin levels in relation with hormonal and metabolic profile in patients with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2014; 176:168–172.
60. Cebeci AN, Guven A, Kuru LI. Perinephric adipose tissue thickness in relation to blood pressure, plasma apelin and C-reactive protein levels in obese adolescents. Minerva Endocrinol. 2016; 41:166–174.
61. Cekmez F, Cekmez Y, Pirgon O, Canpolat FE, Aydinöz S, Metin Ipcioglu O, et al. Evaluation of new adipocytokines and insulin resistance in adolescents with polycystic ovary syndrome. Eur Cytokine Netw. 2011; 22:32–37.
62. Chang CY, Tsai YC, Lee CH, Chan TF, Wang SH, Su JH. Lower serum apelin levels in women with polycystic ovary syndrome. Fertil Steril. 2011; 95:2520–2523. 2523.e1–2523.e2.
63. Choi YS, Yang HI, Cho S, Jung JA, Jeon YE, Kim HY, et al. Serum asymmetric dimethylarginine, apelin, and tumor necrosis factor-alpha levels in non-obese women with polycystic ovary syndrome. Steroids. 2012; 77:1352–1358.
64. Tapan S, Tascilar E, Abaci A, Sonmez A, Kilic S, Erbil MK, et al. Decreased plasma apelin levels in pubertal obese children. J Pediatr Endocrinol Metab. 2010; 23:1039–1046.
65. Principe A, Melgar-Lesmes P, Fernández-Varo G, del Arbol LR, Ros J, Morales-Ruiz M, et al. The hepatic apelin system: a new therapeutic target for liver disease. Hepatology. 2008; 48:1193–1201.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download