Abstract
Epithelioid trophoblastic tumor (ETT) is a rare gestational trophoblastic neoplasm composed of intermediate trophoblasts. Most cases of ETT are reported in women of reproductive age following a prior gestation within 2 weeks to 30 years. ETT is extremely rare in postmenopausal women. It is commonly misdiagnosed as a poorly differentiated carcinoma or another type of gestational trophoblastic tumor. We report a case of ETT in a 56-year-old woman that developed 23 years after the patient's last pregnancy.
Epithelioid trophoblastic tumors (ETT) are a rare form of gestational trophoblastic neoplasm (GTN) that is composed of a monomorphic population of chorionic-type intermediate trophoblastic cells.1 Rarely, trophoblastic disease may develop independently of gestation in postmenopausal women.23
Here, we describe a case of ETT in a 56-year-old woman that developed 23 years after the patient's last pregnancy.
A 56-year-old Korean woman (gravida 2, para 1) presented with a history of vaginal bleeding, which had started 3 months earlier. She was healthy at birth and had no history of an underlying disease or a clotting abnormality. In addition, there was no history of oral medication, pregnancy, or any other gynecological/surgical problem. She was postmenopausal for the past 10 years and her last childbirth was 23 years prior.
Laboratory results, including hemoglobin, coagulation panel, and biochemical tests, did not reveal any data of interest. Her serum cancer antigen 125 (CA-125) level was normal and other tumor markers were not performed preoperatively. A Pap smear taken prior to surgery was normal. Ultrasonography revealed a thin endometrium and showed otherwise normal findings with no free fluid.
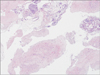
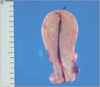
A dilatation and curettage revealed blood clots, decidua with necrosis, and nodular proliferation of epithelioid trophoblasts (Fig. 1). A total abdominal hysterectomy with bilateral salpingo-oophorectomy subsequently performed showed a slightly enlarged uterus (Fig. 2). The pathology demonstrated ETT.
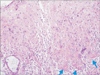
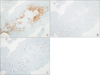
On microscopic examination, there was an epithelial-like growth pattern. The tumor was composed of nests and cords, and had an expansive infiltrative pattern. The neoplastic cells had a moderate degree of pleomorphism and large eosinophilic cytoplasm, some of which had prominent nucleoli. The tumor showed cell necrosis with hyaline-like material, resembling a squamous cell carcinoma (Fig. 3). Immunohistochemistry showed a diffuse positive staining for P63, placental alkaline phosphatase (PLAP), and was highly positive for Ki67 (Fig. 4).
Postoperatively, serum β-human chorionic gonadotropin (β-hCG) level was < 0.8 mIU/mL (normal < 2 mIU/mL). A repeated serum β-hCG analysis was negative for 3 weeks. Chest radiographs and computed tomography (CT) scans of the chest, abdomen, and pelvis showed normal finding. The patient did not receive chemotherapy. At the time of reporting, the patient was alive with no evidence of disease for 22 months after the surgery.
ETT is a rare tumor composed of intermediate trophoblasts. It occurs mostly during reproductive age with a mean age of onset at 36.1 years.1 It is extremely rare in postmenopausal women.4
ETT was first characterized in 1998 by Drs. Shih and Kurman,1 who recognized it as a rare form of trophoblastic disease composed of intermediate trophoblast cells with features that made it distinct from placental site trophoblastic tumor (PSTT) and choriocarcinoma. The intermediate trophoblast is thought to have a phenotype that is between that of the primitive cytotrophoblast and the terminally differentiated syncytiotrophoblast. The intermediate trophoblast is the primary cell type in an exaggerated placental site, placental site nodule, PSTT, and ETT. Based on the anatomic location in the pregnant uterus, intermediate trophoblasts can be divided into villous, implantation site, and chorionic subtypes. The placental chorionic-type intermediate trophoblast has a nodular growth pattern and is thought to be the cell type of origin for lesions with a similar growth pattern, including placental site nodule and ETT. In contrast, the placental implantation site intermediate trophoblast tends to have an infiltrative growth pattern, as does its neoplastic counterpart, PSTT. ETTs are considered a neoplasm composed of chorionic-type intermediate trophoblasts based on histologic characteristics, immunohistochemical expression, and polymerase chain reaction analysis.156
ETT is usually associated with a prior gestational event. These antecedent gestational events include full-term deliveries, spontaneous abortion, hydatidiform mole, and choriocarcinoma. The interval between the preceding gestational event and the diagnosis of ETT ranges from 1 to 30 years.1 Our patient had a full-term delivery 23 years before the diagnosis.
Serum β-hCG levels in most cases of ETT are raised.1 A high level of serum β-hCG in ETT is associated with a large mass and high mitotic activity.7 The low level of serum β-hCG in our patient may have been due to a small tumor volume or may have been a postoperative result.
As eosinophilic hyaline-like material resembling keratin is an integral part of ETT, ETT can be easily misinterpreted as a keratinizing squamous cell carcinoma of the cervix.1 Several cases have been reported that were initially misdiagnosed as cervical squamous carcinomas because of in situ cervical spread.8 Extra-uterine ETT can be especially difficult to diagnosis if it presents as a metastatic lesion. If the lesion was located in the ovary and distant lymph node, other malignant disease should be excluded.910
The differential diagnosis of ETT includes other GTNs, such as choriocarcinoma and PSTT. Immunohistochemical studies can help differentiate ETT from choriocarcinoma and PSTT. In ETT, both β-hCG and human placental lactogen (hPL) exhibit focal reactivity.11 This is in distinct contrast to the staining patterns seen in choriocarcinoma and PSTT. In choriocarcinoma, β-hCG is diffusely positive, highlighting numerous syncytiotrophoblastic cells, whereas hPL positive cells are less conspicuous. The converse is typical of PSTT, in which there is diffuse positive staining for hPL while staining for β-hCG tends to be focal or absent. P63 is expressed in chorionic-type intermediate cells (ETT), but not in PSTT.12
We report this case due to the rarity of trophoblastic tumors in postmenopausal women and the associated diagnostic dilemma. ETT should be considered in the differential diagnosis when the postmenopausal patient presents with vaginal bleeding.
Figures and Tables
Fig. 1
Endometrial biopsy specimen show nodular proliferation of epithelioid trophoblasts (H&E, ×10).
References
1. Shih IM, Kurman RJ. Epithelioid trophoblastic tumor: a neoplasm distinct from choriocarcinoma and placental site trophoblastic tumor simulating carcinoma. Am J Surg Pathol. 1998; 22:1393–1403.
2. Massenkeil G, Crombach G, Dominik S, De Bruyne F, Nitz U, Krussel J, et al. Metastatic choriocarcinoma in a postmenopausal woman. Gynecol Oncol. 1996; 61:432–437.
3. McLellan R, Buscema J, Currie JL, Woodruff JD. Placental site trophoblastic tumor in a postmenopausal woman. Am J Clin Pathol. 1991; 95:670–675.
4. Park SY, Park MH, Ko HS, Cha EJ, Sohn JS, Kim CJ. Epithelioid trophoblastic tumor presenting as an ovarian mass in a postmenopausal woman. Int J Gynecol Pathol. 2014; 33:35–39.
5. Shih IM, Kurman RJ. The pathology of intermediate trophoblastic tumors and tumor-like lesions. Int J Gynecol Pathol. 2001; 20:31–47.
6. Oldt RJ 3rd, Kurman RJ, Shih Ie M. Molecular genetic analysis of placental site trophoblastic tumors and epithelioid trophoblastic tumors confirms their trophoblastic origin. Am J Pathol. 2002; 161:1033–1037.
7. Shet T, Parage M, Maheshwari A, Nair R, Gupta S, Tongaonkar H, et al. Epithelioid trophoblastic tumor of uterus presenting as an ovarian mass: a diagnostic and therapeutic dilemma. Indian J Pathol Microbiol. 2008; 51:242–244.
8. Narita F, Takeuchi K, Hamana S, Ohbayashi C, Ayata M, Maruo T. Epithelioid trophoblastic tumor (ETT) initially interpreted as cervical cancer. Int J Gynecol Cancer. 2003; 13:551–554.
9. Lee CM, Lim S, Cho HY, Lee JS, Shin JW. Sclerosing sromal tumor of the ovary in postmenopausal women: a report of two cases. J Menopausal Med. 2015; 21:115–119.
10. Jeong HJ, Kim HJ, Lee EH, Lee HW, Kim MK. Perimenopausal ovarian carcinoma patient with subclavian node metastasis proven by immunohistochemistry. J Menopausal Med. 2014; 20:43–46.
11. Li J, Shi Y, Wan X, Qian H, Zhou C, Chen X. Epithelioid trophoblastic tumor: a clinicopathological and immunohistochemical study of seven cases. Med Oncol. 2011; 28:294–299.
12. Shih Ie M. Trophogram, an immunohistochemistry-based algorithmic approach, in the differential diagnosis of trophoblastic tumors and tumorlike lesions. Ann Diagn Pathol. 2007; 11:228–234.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download