This article has been corrected. See "Erratum to: Raloxifene Administration in Women Treated with Long Term Gonadotropin-releasing Hormone Agonist for Severe Endometriosis: Effects on Bone Mineral Density" in Volume 23 on page 77.
Abstract
Objectives
To evaluate the efficacy of raloxifene in preventing bone loss associated with long term gonadotropin-releasing hormone agonist (GnRH-a) administration.
Methods
Twenty-two premenopausal women with severe endometriosis were treated with leuprolide acetate depot at a dosage of 3.75 mg/4 weeks, for 48 weeks. Bone mineral density (BMD) was evaluated at admission, and after 12 treatment cycles.
Endometriosis, a common gynecological condition causing cyclical pain, dyspareunia, and infertility, affects 2% to 5% of women of reproductive age.1 Endometriosis commonly recurs after medical or surgical treatment; in the absence of a drug treatment that is safe for long-term use, recurrence can lead to hysterectomy and bilateral oophorectomy.2 Goals of therapy include relief of symptoms, resolution of existing lesions, and prevention of new lesions.3 Gonadotropin-releasing hormone agonist (GnRH-a) is increasingly used to treat endometriosis.4
Therapy with GnRH-a achieves hypoestrogenism and amenorrhea by suppressing pituitary gonadotropin secretion. Several studies have shown that use of GnRH-a promotes subjective and objective improvements in endometriosis related symptoms.456
Prolonged treatment is often required to achieve clinically significant effects on endometriosis symptoms. However, there is concern about the clinical, physical, and biochemical side effects of hypoestrogenism, particularly the rapid reduction in bone mineral density (BMD) that could increase the risk of osteoporosis. Thus, the use of GnRH-a is generally restricted to a 6-month course.78910
GnRH-a related hypoestrogenism frequently causes climacteric-like symptoms, such as vasomotor symptoms and overall severe bone loss.1112 Several drugs have been associated with GnRH-a to reduce these side effects and to allow prolonged treatment.1112131415161718
Raloxifene hydrochloride is a synthetic non-steroidal drug derived from benzothiophene and afferent to selective estrogen receptor modulators (SERMs), a group of compounds that interact with estrogen receptors eliciting tissue-specific responses.1920
Raloxifene acts on the metabolism, central nervous system, skeleton and cardiovascular system as an estrogenic agonist,21222324 whereas it shows a weak estrogenic antagonist effect on reproductive organs, including the breast and uterus.252627 Therefore, raloxifene has been used as a therapeutic agent for osteoporosis.
Continuous administration of GnRH-a inhibits the release of gonadotropins inducing a down-regulation of pituitary GnRH receptors and a state of hypogonadotropic hypogonadism.1112
Thus far, there has been limited research regarding raloxifene administration for the prevention of the bone loss associated with GnRH-a. The aim of this study was to evaluate whether the bone loss that occurs when using GnRH-a could be prevented.
The present study was conducted in the department of obstetrics and gynecology at Chosun University Hospital. From January 2012 to December 2015, 22 reproductive female patients from 25 to 51 years old who suffered from endometriosis were enrolled in a non-randomized retrospective study.
Exclusion criteria were as follows: patients with BMD below the age-matched normal range (z-score below -1.5), who had physical findings that affected the measurement of BMD, such as fracture or osteoarthritis of spine, and who were administered drugs that affected bone turnover, or who received therapy for endometriosis within 4 weeks prior to the start of the study. Written consent from the patient was obtained before enrollment.
Thirty-two patients were enrolled in this study. Four patients discontinued leuprolide administration due to adverse effects or undesirable complications. Two patients were excluded because hormone drugs (oral pill) had been administered within 4 months prior to the start of treatment, and four patients were excluded because BMD was not measured correctly. Thus, the analysis was performed on 22 patients.
At the start of the study, all subjects were randomized in a study design using a computer-generated randomization list. The subjects were assigned to 22 women. All women received leuprolide acetate depot (LAD) (Enantone; Takeda, Rome, Italy) at a dose of 3.75 mg/4 weeks combined with raloxifene hydrochloride (Evista; Eli Lilly, Sesto Fiorentino, Italy) at a dose of 60 mg/day, p.o. The duration of the study was 12 cycles of 4 weeks each, and for this period, the single-blinding was maintained.
BMD was measured at the beginning of the study and after cycle 12 treatment with GnRH-a plus raloxifene.
The BMD was determined using a prodigy series X-ray tube housing assembly (LUNAR, GE Medical system, Madison, WI, USA) at the posterior-anterior lumbar spine (vertebrae L1 to L4) and hip (trochanter, Ward's and femoral neck).
The results of absorptiometry were examined by a single observer blind to different treatment regimens. The primary end-point was the lumbar spine BMD. Hip trochanter, Ward's and femoral neck BMD were considered secondary end-points.
In this study, the paired t-test was used to compare the BMD differences of the lumbar spine, trochanter, femoral neck, and Ward's in women before and 1 year after treatment with GnRH-a plus raloxifene. A P value of < 0.05 indicated statistical significance. Statistical analysis was performed using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA).
Twenty-two enrolled patients completed the study. All the patients had endometriosis diagnosed by laparoscopy. The classification of patients is shown in Table 1.
Subjects in this study had a mean body weight of 62.2 ± 10.2 kg, mean height of 159 ± 5.1 cm, and boyd mass index (BMI) of 24.7 ± 4.3 kg/m2.
We designed a prospective study and checked lumbar spine, trochanter, femoral neck, and Ward's BMD in 22 patients at the start of the study and 1 year after treatment with GnRH-a plus raloxifene.
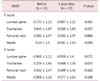
At cycle 12 of treatment with GnRH-a plus raloxifene, lumbar spine, trochanter, femoral neck, and ward's BMD differed from before treatment. The lumbar spine and trochanter decreased slightly at the 1 year after treatment than before (Table 2), but were not significantly different.
Use of GnRH-a has an established place in the medical treatment of endometriosis. These agents act by down-regulating pituitary gonadotropins, resulting in the suppression of gonadotropin production and secondary ovarian suppression. This ovarian suppression may lead to osteopenia by reducing the BMD.
Accordingly, treatment with GnRH-a alone is generally restricted to 6 month duration because of concern about continuing loss of BMD over longer treatment periods. All studies agree that bone loss occurs with continued use of GnRH-a for 6 months or more.
Circulating estradiol levels decrease to the post-menopausal range, producing important menopausal side effects, such as a reduction in BMD at the lumbar spine2829 and proximal femur.30 Therefore, there is a need for the prevention of bone loss during long term treatment with GnRH-a.
Raloxifene at the standard dosage of 60 mg daily prevents postmenopausal bone loss in women without osteoporosis and is used also to treat established postmenopausal osteoporosis.3132
In addition, raloxifene reduces the risk of vertebral fractures in postmenopausal osteoporotic women with or without preexisting fractures by about 40% versus a placebo,22333435 thereby improving quality of life.36
In women treated with GnRH-a, the positive effect of raloxifene on bone metabolism was also confirmed by the lack of significant change in biochemical parameters of bone formation and reabsorption.37 Goldstein et al.27 confirmed that raloxifene did not induce endometrial proliferation in post-menopausal women, unlike estrogen.
Besides, a variety of anti-reabsorptive drugs has been used to preserve the bone tissue during GnRH-a treatment. During our study period, few side effects were detected and raloxifene treatment was tolerated.
In the case of severe endometriosis, patients were administered GnRH-a for 6 months. However, sometimes the pain recurred after normal menstruation restarted. Therefore, a method of treating patients has not been fully established.
If necessary, the treatment could be administered in the oral pill, progestin but GnRH-a is known to suppress endometrial lesions and symptoms. Therefore, GnRH-a was administered with raloxifene to inhibit bone loss that was the greatest side effect of GnRH-a administration.
In a previous report, there was no change in BMD when a combination of GnRH-a and raloxifene was administered for six months, thereby the present study administered a combination of GnRH-a and raloxifene for 1 year in patients who suffered from pain.
BMD continues to decline rapidly during the early postmenopausal years.
The annual rates of loss during these intervals were approximately 1.8% to 2.3% in the spine and 1.0% to 1.4% in the hip.38
Our findings suggest that BMD is reduced slightly over 1 year of treatment with GnRH-a and raloxifene, but was not statistically significantly different from the start of the study. Therefore, this treatment does not seem to affect bone loss significantly.
Some limitations of this study deserve mention. It has mostly stemming from a small number of sample, the absence of a control group, and retrospective design. Besides, fracture risk was not assessed.
In conclusion, our study shows that the administration of GnRH-a plus raloxifene in pre-menopausal women with severe endometriosis would be effective to prevent bone loss for long-term treatment. It is possible that raloxifene could be used as an ‘add-back therapy’ in women treated with GnRH-a.
References
1. Haney AF. The pathogenesis and etiology of endometriosis. In : Thomas EJ, Rock JA, editors. Modern approaches to endometriosis. Dordrecht, NL: Kluwer Academic;1991. p. 113–128.
2. Howell R, Edmonds DK, Dowsett M, Crook D, Lees B, Stevenson JC. Gonadotropin-releasing hormone analogue (goserelin) plus hormone replacement therapy for the treatment of endometriosis: a randomized controlled trial. Fertil Steril. 1995; 64:474–481.
3. McLachlan RI, Healy DL, Burger HG. Clinical aspects of LHRH analogues in gynaecology: a review. Br J Obstet Gynaecol. 1986; 93:431–454.
4. Erickson LD, Ory SJ. GnRH analogues in the treatment of endometriosis. Obstet Gynecol Clin North Am. 1989; 16:123–145.
5. Meldrum DR, Chang RJ, Lu J, Vale W, Rivier J, Judd HL. “Medical oophorectomy” using a long-acting GNRH agonist--a possible new approach to the treatment of endometriosis. J Clin Endocrinol Metab. 1982; 54:1081–1083.
6. Lemay A, Maheux R, Faure N, Jean C, Fazekas AT. Reversible hypogonadism induced by a luteinizing hormone-releasing hormone (LH-RH) agonist (Buserelin) as a new therapeutic approach for endometriosis. Fertil Steril. 1984; 41:863–871.
7. Fogelman I. Gonadotropin-releasing hormone agonists and the skeleton. Fertil Steril. 1992; 57:715–724.
8. Lemay A, Surrey ES, Friedman AJ. Extending the use of gonadotropin-releasing hormone agonists: the emerging role of steroidal and nonsteroidal agents. Fertil Steril. 1994; 61:21–34.
9. Orwoll ES, Yuzpe AA, Burry KA, Heinrichs L, Buttram VC Jr, Hornstein MD. Nafarelin therapy in endometriosis: long-term effects on bone mineral density. Am J Obstet Gynecol. 1994; 171:1221–1225.
10. Crosignani P, Olive D, Bergqvist A, Luciano A. Advances in the management of endometriosis: an update for clinicians. Hum Reprod Update. 2006; 12:179–189.
11. Surrey ES. Steroidal and nonsteroidal “add-back” therapy: extending safety and efficacy of gonadotropin-releasing hormone agonists in the gynecologic patient. Fertil Steril. 1995; 64:673–685.
12. Pickersgill A. GnRH agonists and add-back therapy: is there a perfect combination? Br J Obstet Gynaecol. 1998; 105:475–485.
13. Palomba S, Affinito P, Tommaselli GA, Nappi C. A clinical trial of the effects of tibolone administered with gonadotropin-releasing hormone analogues for the treatment of uterine leiomyomata. Fertil Steril. 1998; 70:111–118.
14. Palomba S, Affinito P, Di Carlo C, Bifulco G, Nappi C. Long-term administration of tibolone plus gonadotropin-releasing hormone agonist for the treatment of uterine leiomyomas: effectiveness and effects on vasomotor symptoms, bone mass, and lipid profiles. Fertil Steril. 1999; 72:889–895.
15. Di Carlo C, Palomba S, Tommaselli GA, Guida M, Di Spiezio Sardo A, Nappi C. Use of leuprolide acetate plus tibolone in the treatment of severe premenstrual syndrome. Fertil Steril. 2001; 75:380–384.
16. Palomba S, Pellicano M, Affinito P, Di Carlo C, Zullo F, Nappi C. Effectiveness of short-term administration of tibolone plus gonadotropin-releasing hormone analogue on the surgical outcome of laparoscopic myomectomy. Fertil Steril. 2001; 75:429–433.
17. Taskin O, Yalcinoglu AI, Kucuk S, Uryan I, Buhur A, Burak F. Effectiveness of tibolone on hypoestrogenic symptoms induced by goserelin treatment in patients with endometriosis. Fertil Steril. 1997; 67:40–45.
18. Adashi EY. Long-term gonadotrophin-releasing hormone agonist therapy: the evolving issue of steroidal ‘add-back’ paradigms. Hum Reprod. 1994; 9:1380–1397.
19. Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997; 138:863–870.
20. Khovidhunkit W, Shoback DM. Clinical effects of raloxifene hydrochloride in women. Ann Intern Med. 1999; 130:431–439.
21. Walsh BW, Kuller LH, Wild RA, Paul S, Farmer M, Lawrence JB, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998; 279:1445–1451.
22. Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999; 282:637–645.
23. Mosca L. Rationale and overview of the Raloxifene Use for the Heart (RUTH) trial. Ann N Y Acad Sci. 2001; 949:181–185.
24. Barrett-Connor E, Grady D, Sashegyi A, Anderson PW, Cox DA, Hoszowski K, et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA. 2002; 287:847–857.
25. Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999; 281:2189–2197.
26. Cohen FJ, Watts S, Shah A, Akers R, Plouffe L Jr. Uterine effects of 3-year raloxifene therapy in postmenopausal women younger than age 60. Obstet Gynecol. 2000; 95:104–110.
27. Goldstein SR, Scheele WH, Rajagopalan SK, Wilkie JL, Walsh BW, Parsons AK. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000; 95:95–103.
28. Dawood MY, Lewis V, Ramos J. Cortical and trabecular bone mineral content in women with endometriosis: effect of gonadotropin-releasing hormone agonist and danazol. Fertil Steril. 1989; 52:21–26.
29. Whitehouse RW, Adams JE, Bancroft K, Vaughan-Williams CA, Elstein M. The effects of nafarelin and danazol on vertebral trabecular bone mass in patients with endometriosis. Clin Endocrinol (Oxf). 1990; 33:365–373.
30. Stevenson JC, Lees B, Gardner R, Shaw RW. A comparison of the skeletal effects of goserelin and danazol in premenopausal women with endometriosis. Horm Res. 1989; 32:Suppl 1. 161–163. discussion 4.
31. Clemett D, Spencer CM. Raloxifene: a review of its use in postmenopausal osteoporosis. Drugs. 2000; 60:379–411.
32. Díez JL. Skeletal effects of selective oestrogen receptor modulators (SERMs). Hum Reprod Update. 2000; 6:255–258.
33. Prestwood KM, Gunness M, Muchmore DB, Lu Y, Wong M, Raisz LG. A comparison of the effects of raloxifene and estrogen on bone in postmenopausal women. J Clin Endocrinol Metab. 2000; 85:2197–2202.
34. Lufkin EG, Whitaker MD, Nickelsen T, Argueta R, Caplan RH, Knickerbocker RK, et al. Treatment of established postmenopausal osteoporosis with raloxifene: a randomized trial. J Bone Miner Res. 1998; 13:1747–1754.
35. Meunier PJ, Vignot E, Garnero P, Confavreux E, Paris E, Liu-Leage S, et al. Treatment of postmenopausal women with osteoporosis or low bone density with raloxifene. Raloxifene Study Group. Osteoporos Int. 1999; 10:330–336.
36. Silverman SL, Minshall ME, Shen W, Harper KD, Xie S. The relationship of health-related quality of life to prevalent and incident vertebral fractures in postmenopausal women with osteoporosis: results from the Multiple Outcomes of Raloxifene Evaluation Study. Arthritis Rheum. 2001; 44:2611–2619.
37. Palomba S, Orio F Jr, Morelli M, Russo T, Pellicano M, Nappi C, et al. Raloxifene administration in women treated with gonadotropin-releasing hormone agonist for uterine leiomyomas: effects on bone metabolism. J Clin Endocrinol Metab. 2002; 87:4476–4481.
38. Finkelstein JS, Brockwell SE, Mehta V, Greendale GA, Sowers MR, Ettinger B, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008; 93:861–868.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download