Abstract
Objectives
This report seeks to introduce some cases of the patients who received magnetic resonance imaging (MRI)-guided high intensity focused ultrasound (HIFU) surgery (MRgFUS)-based intramural uterine fibroids treatment where the post-MRgFUS intramural uterine fibroids decreased in its volume and protruded towards the endometrial cavity to be expelled by hysteroscopy.
Methods
Of the 157 patients who had received MRgFUS treatment in the Obstetrics and Gynecology of the Hospital from March, 2015 to February, 2016; this study examined 6 of the cases where, after high intensity focused ultrasound treatment, intramural uterine fibroids protruded towards the endometrial cavity to be removed by hysteroscopic myomectomy. The high intensity focused ultrasound utilized in the cases were Philips Achieva 1.5 Tesla MR (Philips Healthcare, Best, The Netherlands) and Sonalleve HIFU system.
Results
The volume of fibroids ranged from 26.0 cm3 to 199.5 cm3, averaging 95.6 cm3. The major axis length ranged from 4.0 cm to 8.2 cm, averaging 6.3 cm. Fibroid location in all of the patients was in intramural uterine before treatment but after the high intensity focused ultrasound treatment, the fibroids were observed to protrude towards the endometrial cavity in at least Day 5 or up to Day 73 to allow hysteroscopic myomectomy.
Fibroid is a common disease in women and accompanies multiple symptoms such as menstrual pain and profuse menstruation.1 For the ultimate cure of fibroids, several operational procedures have been developed such as hysterectomy or minimally invasive surgical procedures continuously including abdominal myomectomy, laparoscopic myomectomy, uterine artery embolization, and fibroids amylolysis. Recently, in the widely-operated single incision laparoscopic surgery, the number of ports used through the abdominal cavity was reduced to one from the 3 to 4 before, maximizing the aesthetic effect.23 However, the procedure requires general anesthesia, 4 to 5 day hospitalization period, and relatively longer period of recovery in this busy modern society. For this reason, more non-invasive treatment methods have been sought after. Recently, as a non-invasive method, high intensity focused ultrasound (HIFU) was introduced and utilized in many different ways. For menopausal women, most of their fibroids tend to decrease after menopausal and their symptoms are recovered. Given this fact, HIFU is regarded as a very promising treatment. The HIFU treatment is a kind of thermotherapy. By instantly raising the temperature of the focus area up to 57℃ to 80℃, the method induces coagulative meronecrosis. It does not require general anesthesia and hospitalization while preserving the uterus. Patients can leave the hospital on that same day and return to their normal life.
The HIFU can be largely divided into ultrasound image guided HIFU (USgFUS) and magnetic resonance imaging (MRI) guided HIFU (MRgFUS) based on the method of image guide during operation. After the HIFU treatment, fibroids tend to be fast reduced for the first 6 months. Depending upon the reduction in their sizes, the degree of symptom recovery varies.4 However, the HIFU treatment cannot remove fibroids completely and tissue inspection is difficult.
Among the patients who received intramural uterine fibroids expulsion based on the MRgFUS in the Obstetrics and Gynecology (Ob/Gyn) of the Hospital, some of their intramural uterine fibroids decreased in their volumes after MRgFUS treatment and protruded towards the endometrial cavity to be removable by hysteroscopy. This report will introduce such cases.
This report introduces 6 cases where intramural uterine fibroids protruded toward the endometrial cavity after HIFU treatment to become removable by hysteroscopic myomectomy among the 157 patients who had received MRgFUS treatment in the Ob/Gyn of the Hospital from March 2015 to February 2016. This study was approved by the institutional review board (IRB) of this hospital. All of the study treatment procedures were performed by the HIFU team consisting of 4 obstetricians, 2 medical imaging specialists, 2 radiotherapy specialists and 1 nurse. Patients’ information was collected via retrospective data analysis based on hospitalization record, outpatient record, and operational record. Patients’ information includes age, past medical operation history, indicant of HIFU treatment, fibroid sizes and volumes, fibroids Funaki type, duration of HIFU treatment, non-perfused volume (NPV) ratio after HIFU treatment, difference in pre/post-treatment blood test, post-operational pain and skin burn, intestinal injury, bladder injury, endometrial damage or other complication existence, clinical symptom change in post-treatment Month 1, and the period to hysteroscopic myomectomy implementation. The MRI and HIFU used in this research were Philips Achieva 1.5 Tesla MR (Philips Healthcare, Best, The Netherlands) and Sonalleve HIFU system. If a fibroid showed low intensity image compared with the pre-treatment T2-weighted image, such a case was defined as Type I; higher than the skeletal muscle signal but lower than myometrium signal, Type II; equal to or higher than the myometrium signal, Type III.5 To evaluate the seriousness of patients’ symptoms, the symptom severity score (SSS) was utilized in the study.6 Treatment duration was defined as the time of HIFU treatment commencement to the time of final round of HIFU treatment. For pre/post-treatment blood tests, the measurements gained in the last pre-treatment blood test were utilized as well as the numbers in post-treatment Month 1. Complication is defined as a physical status in need of additional medical treatment due to HIFU treatment. Such cases include urogenital problems such as hematuria; at least 3-day-long fever, intestinal injury and skin burn. Temporary hematuria and skin burn not more serious than the first degree and not larger than 1cm in its size were excluded.
All of the patients fasted for 8 hours then visited the outpatient unit to do the general preparation for surgical operation and moved to the MRI room. The patients took a prone position on the MRI bed. HIFU coils were placed on the patients and do MRI scan to adjust a precise focus. If the intestine was found in front of uterus in the first MRI scan test just before the treatment, Modified BRP technique was conducted to avoid possible intestinal injury by high intensity ultrasound. Modified BRP technique is to change the position of uterus or intestine by filling in the bladder with normal saline or filling in the rectum with glycerin or through other diverse techniques in order not to expose intestine to HIFU for intestine damage prevention. For the sake of safety, an emergency stop button was placed in the patients’ hand. Then, HIFU treatment was done under Quasi real-time MRI scanning. Based on thermometry, HIFU-caused temperature change in the focus area was observed along with the temperatures of neighboring organs (Fig. 1). Every patient received pre-operational T2-, T1-weighted MRI and contrast enhanced T1-weighted MRI scanning. Instantly after the operation, they were scanned with the contrast enhanced T1-weighted MRI to check the non-perfused volume (NPV) before completion (Fig. 2). Post-treatment NPV was calculated by measuring the circumference in sequential slice.4
The patients were observed on post-treatment Day 1, Day 7, in Month 1, Month 3, and Month 6. If their fibroids were found to protrude toward the endometrial cavity in ultrasound images, hysteroscopic myomectomy was implemented for histological inspection (Fig. 3).78 Only one of the cases showed persistent uterine bleeding.
In the 6 cases of hysteroscopic myomectomy which showed post-treatment intramural uterine fibroid projection toward the endometrial cavity, the patients’ ages ranged widely from 30 to 45, averaging 39.17 years old (Table 1). Two of them were found to have previous medical operation record and received hysteroscopic myomectomy and laparoscopic myomectomy. All of the patients were before menopausal and three of them experienced child delivery. In all patients, fibroids were located in intramural uterine and deemed difficult to remove by hysteroscopy in ultrasound examination. The indicants of HIFU treatment were observed profuse menstruation and menstrual pain in all of the patients. Fibroid volumes ranged from 26.0 cm3 to 199.5 cm3 to average 95.6 cm3; and major axis lengths were from 4.0 cm to 8.2 cm to average 6.3 cm. Fibroid Funaki type of Case 1 was Type I; Case 4, Type II; and Case 1, Type III. Case 1 had a single fibroid and the other Cases had multiple fibroids ranging from 2 to 5. The power of HIFU was between 80 W and 140 W to average 101.7 W/cm2. NPV ratio was from 81.6% to 96.2%, averaging 88.4%. SSS ratio change in Month 1 was 65.2; Month 3, 35.4; and Month 6, 8.3 to show clear recovery of the symptom. Pre-treatment hemoglobin levels were between 6.1 g/dL and 13.2 g/dL to average 11.1 g/dL. In post-operational Month 1, the average was 13.2 ± 0.7 showing increase. In all of the patient cases, no transfusion was conducted. Four patients showed post-operational uterine hemorrhage. In Case 1, cancer antigen 125 (CA-125) was 51.7 before the treatment but fell to 17.2 in post-operational Month 1. Pre-treatment fibroid locations in all patients were intramural uterine but on at least day 5 or up to day 73 after the HIFU treatment, their fibroids were observed to protrude toward the endometrial cavity in the ultrasound exam or MRI scan. Thus, under the abdominal ultrasound supervision, hysteroscopic myomectomy was implemented. No endometrium damage regarding hysteroscopy was observed.
Most fibroids reduce in their sizes after menopausal and the symptoms tend to be eased in many cases. For this reason, many menopausal women are reluctant to receive a surgical operation regarding this gynecological disease of fibroid. There are diverse non-invasive measures against fibroids. Recently, as the Korean Ministry of Food and Drug Safety established the guidelines for the evaluation of HIFU operation in 2009, the HIFU treatment has begun to be widely recognized and discussed.9 The guidelines regulate that HIFU should raise temperature of focus area tissue no lower than 55℃ and cause no larger temperature change than 10℃ in areas other than the focus area. It should have an acoustic intensity at least 1000 W/cm2 in focus area and should have a transducer for medical treatment accompanying linear and rotating movement range. The existing traditional thermotherapy temperature should not exceed 45℃ so, it is difficult to bring about coagulative necrosis. And the possibility of recurrence is relatively high.10 The HIFU treatment is a different method from the existing tumorous thermotherapy. It is to raise the temperature of tissues in focus area up to 60℃ to 80℃ shortly to destroy the diseased tissues and blood vessels.11 This coagulative necrosis is strictly limited to the focus area, minimizing normal tissue damage. Since the blood temperature rises fast in the area and the transferred thermal energy is lost along the blood stream, only microvessels or relatively fine vessels are destroyed while leaving great vessels undamaged.1213 Such a selective way of destruction is clinically significant. Intra-tumor nutrition supply through blood vessels is directly destroyed and the treatment stability is effectively guaranteed. Aradhana et al. investigated 11 patients receiving hysterectomy within 30 days from MRgFUS treatment. They reported that the NPV area in MRI image and expected heat-affected area were not different from the necrosis area and heat-affected area in the histologic examination.14 MRgFUS complications are known to include skin burn, thermal damage in abdominal wall muscle and subcutaneous fat, sciatic nerve damage, hematuria, intestinal injury, abnormal uterine bleeding and others. But many studies have hardly reported damage in neighboring organs whereas commonly reported minor skin burns. It is deemed that this is thanks to the assistance of MRI supervision through thermometry about thermal diffusion into adjacent organs.15 For this reason, MRgFUS studies have focused on the treatment effectiveness and re-intervention due to fibroid recurrence.
Yoon et al.16 examined 29 patients in their study and reported their symptoms were recovered thanks to NPV ratio 42.1 ± 17.6% treatment by 83% in 3 months; and 90% in 6 months. Anne et al. utilized the equipment identical to the Hospital’s HIFU equipment and they examined 36 patients for 21.4 months on average. As a result, they found 61.1% showed clinical effectiveness and reported re-intervention in 12, 18, and 24 months was 2.8%, 8.5%, and 21.6%, respectively.17 Morita et al.18 followed up 48 patients receiving HIFU treatment for 6 months; then reported 33% reduction in their fibroid volumes, indicating a significant correlation with P value 0.037 between the NPV ration right after the operation and fibroid volume reduction. In their 12-month follow-up, 15% showed dissatisfaction with the treatment and 45% required a surgical procedure. Funaki et al.4 followed up 91 patients who had received HIFU treatment for 24 months. They found significant volume reduction (−36.5%) in Types I and II fibroids in over 50% of the NPV ratio patients for initial 6 months after HIFU treatment. For the subsequent 6 to14 months, minor volume reduction (−39.5%) was reported. Type III fibroids were reported to have shown no significant reduction for the initial 6 months (−9.1%) while slightly decreasing for the following 24 months. However, they also stated that every patient’s symptoms were relieved as they showed significant SSS reduction within the first 3 months after the HIFU treatment. They mentioned that 14% of Types I and II cases and 21.6% of Type III cases had received a surgical procedure or re-intervention such as second round of HIFU treatment.3 Quinn et al.19, in their study on 5-year follow-up after MRI HIFU treatment, reported the re-intervention ratio at 42.8% for 3 years; and up to 58.6% for 5 years, arguing that it was related to post-treatment NPV ratio. However, these studies also did not report the kinds of re-intervention in details.
This present study introduced 6 cases of clinical experience where, after HIFU treatment under the guidance of magnetic resonance image, fibroids were reduced in their size and protruded toward the endometrial cavity to be removed by using hysteroscopy.
Preceding studies mainly described fibroid volume reduction and factors affecting thereon. But no study has reported a case where fibroids were projected toward the endometrial cavity after HIFU treatment to invite another procedure. In this sense, the present study is all the more meaningful. It is deemed for the author of this study that when fibroid sizes decrease, the part located on the myometrium side shrinks more than the part on the endometrium side to protrude toward the endometrial cavity. It is desirable to analyze more case examples and find out factors to predict the potential movement of an intramural uterine fibroid toward the endometrial cavity after HIFU treatment.
Figures and Tables
Fig. 1
T2-weighted images I the coronal (A) and sagittal (B) plane taken during magnetic resonance imaging-guided high intensity focused ultrasound surgery (MRgFUS) treatment. The cells are round in the transverse plane (A) and have an ellipsoid shape in their longitudinal direction (B). This system is possible to show the thermometry and monitor all the intraabdominal organ regardless depth. The crossed white lines show the pathway of the ultrasound beam. White and orange lines shown in demark the border of the region show certain (white) thermal damage or potential (orange) thermal damage.

Fig. 2
Intramural type of myoma before magnetic resonance imaging (MRI)-guided high intensity focused ultrasound surgery (MRgFUS) was changed to submucosal type after MRgFUS treatment on the MRI finding, (A) Intramural myoma before MRgFUS treatment, (B) Immediate intramural myoma after MRgFUS treatment, (C) Myoma protruding to endometrial cavity in the 3 month later after MRgFUS treatment.

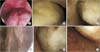
Fig. 3
(A-F) The various cases with myoma protruding to the endometrial cavity after magnetic resonance imaging-guided high intensity focused ultrasound surgery (MRgFUS) treatment in hysteroscopic finding, not before.
References
1. Choi SJ, Lee YY, Kim JH, Choi DS, Bae DS, Yoon BK. The effects of hormonal therapy on uterine leiomyomas in perimenopausal women. J Korean Soc Menopause. 2004; 10:269–276.
2. Kim YW. Single port transumbilical total laparoscopic hysterectomy (TLH): initial experience in Korea. Korean J Obstet Gynecol. 2009; 52:480–486.
3. Yoon BS, Park H, Seong SJ, Park CT, Park SW, Lee KJ. Single-port laparoscopic salpingectomy for the surgical treatment of ectopic pregnancy. J Minim Invasive Gynecol. 2010; 17:26–29.
4. Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol. 2009; 34:584–589.
5. Funaki K, Fukunishi H, Funaki T, Sawada K, Kaji Y, Maruo T. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am J Obstet Gynecol. 2007; 196:184.e1–184.e6.
6. Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz-Murphy K, Gonzalves SM. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol. 2002; 99:290–300.
7. Uy K, Kim TH, Lee HH, Chung SH, Lee BI. Clinical applications of hysteroscopic surgery in postmenopausal women. J Korean Soc Menopause. 2010; 16:46–51.
8. Lee HS, Cho YJ, Kim JM. Risk factors associated with premalignant and malignant endometrial polyps. J Korean Soc Menopause. 2013; 19:74–80.
9. National Institute of Food and Drug Safety Evaluation. High intensity focused type ultrasound operator (HIFU) assessment guideline. Seoul: National Institute of Food and Drug Safety Evaluation;2009.
10. Yao-long L, Chun-ying W, Kuo W. Therapeutic effect of HIFU treatment for benign and malignant solid tumor. Chin J Med Imaging. 2007; 15:106–112.
11. Stewart EA, Gedroyc WM, Tempany CM, Quade BJ, Inbar Y, Ehrenstein T, et al. Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol. 2003; 189:48–54.
12. Stewart EA, Rabinovici J, Tempany CM, Inbar Y, Regan L, Gostout B, et al. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril. 2006; 85:22–29.
13. Hindley J, Gedroyc WM, Regan L, Stewart E, Tempany C, Hynyen K, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: early results. AJR Am J Roentgenol. 2004; 183:1713–1719.
14. Venkatesan AM, Partanen A, Pulanic TK, Dreher MR, Fischer J, Zurawin RK, et al. Magnetic resonance imaging-guided volumetric ablation of symptomatic leiomyomata: correlation of imaging with histology. J Vasc Interv Radiol. 2012; 23:786–794.e4.
15. McDannold N, Tempany C, Jolesz F, Hynynen K. Evaluation of referenceless thermometry in MRI-guided focused ultrasound surgery of uterine fibroids. J Magn Reson Imaging. 2008; 28:1026–1032.
16. Yoon SW, Kim KA, Hwang YY, Lee C, Cha SH, Lee SY, et al. Magnetic resonance imaging-guided focused ultrasound surgery for uterine fibroids: Initial experience in Korea. Korean J Obstet Gynecol. 2008; 51:982–987.
17. Thiburce AC, Frulio N, Hocquelet A, Maire F, Salut C, Balageas P, et al. Magnetic resonance-guided high-intensity focused ultrasound for uterine fibroids: Mid-term outcomes of 36 patients treated with the Sonalleve system. Int J Hyperthermia. 2015; 31:764–770.
18. Morita Y, Ito N, Hikida H, Takeuchi S, Nakamura K, Ohashi H. Non-invasive magnetic resonance imaging-guided focused ultrasound treatment for uterine fibroids-early experience. Eur J Obstet Gynecol Reprod Biol. 2008; 139:199–203.
19. Quinn SD, Vedelago J, Gedroyc W, Regan L. Safety and five-year re-intervention following magnetic resonance-guided focused ultrasound (MRgFUS) for uterine fibroids. Eur J Obstet Gynecol Reprod Biol. 2014; 182:247–251.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download