Abstract
Objectives
It has been known that there is a difference in anogenital distance (AGD) in the animals and newborn depending on the exposure of androgenic hormones. The anatomical changes occur in the female genitalia in women after menopause. This was pilot study to find out whether the menopause affects AGD.
Methods
We evaluated a total of 50 women targeted for premenopausal and postmenopausal group in each 25 people. AGD was defined as a length between the posterior commissure of labia and anal center. AGD was measured in lithotomy position using sterile paper ruler. In order to control bias of the height and weight, which could influence the AGD, anogenital index (AGI) is defined as the weight divided by the AGD value. We used a Mann-Whitney U test to analyze the relationship between AGD and menopause for statistical analysis.
The anogenital distance (AGD) is known to vary depending on the exposure to male sex hormone in animals and newborn infants.1,2 Anatomical changes in female genital organs may also occur due to the hormonal changes following menopause in adult females.3
This is a pilot study aimed to identify if menopause affects AGD on a total of 50 subjects including 25 pre-menopausal women and 25 post-menopausal women. AGD is defined as the length between the posterior commissure of labia and the center of the anus. It was measured using a paper ruler in the lithotomy position. The anogenital index (AGI) was used to control two variables of height and weight. AGI was calculated by dividing AGD by body mass index (BMI).
There have been a lot of studies on female external genitalia, but a few on AGD; there are a few studies of AGD in female adults and the anatomical baseline is not determined.3 On the other hands, AGD of newborn infants or adult males has been often investigated. If a fetus is exposed to exogenous androgen or estrogen, the exposure may cause the change in AGD, which is used as a very sensitive metric.4 As stated above, it is already well known that the change of AGD takes place as a fetus is affected by hormones during pregnancy, but whether it occurs even in adults or not is yet discovered. Adult females experience a dramatic change in hormones after menopause. A drop in estrogen affects the female genital organs such as the uterus, vagina, and ovary size. However since few study was conducted on the structural change in the pudendum, this study tried to confirm the correlation between the change in the length from the anus to the vagina and menopause.
The subjects are women who visited the gynecology division of a hospital and agreed on participation after listening to explanations about the study and they were divided into two groups: the pre-menopausal group and the post-menopausal group. Women included in the pre-menopausal group were those between 20 and 45 years of age who menstruated at intervals of 20 to 40 days for the past one year; and women included in the post-menopausal group were those aged 56 or older without vagina bleeding for the past one year. The exclusion criteria are as follows:
1) If she has received pudendum and vaginal surgeries in the past
2) If she is likely to be affected by female sex hormone due to the surgery of pelvic uterine appendages such as oophorectomy
3) If she had congenital anatomical abnormalities in genital organs including mullerine agenesis
4) If she has received treatments to control the secretion of hormones including taking birth control pills, administering gonadotropin releasing hormone, and hormone replacement therapy (HRT)
5) If she did not agree on participation in or is unable to cooperate with the study and if she is mentally ill or refused to participate in the study
6) If she is pregnant
The candidates for subjects were selected according to the criteria above and divided into two groups of 25 pre-menopausal women and 25 post-menopausal women using a random sampling.
Height, weight, and AGD of the study subjects were measured on the same day. Height was measured by rounding off to the nearest tenth in centimeter (cm). Weight was measured by rounding off to the nearest tenth in kilogram (kg). BMI (m/kg2) was calculated by dividing the measured weight in kilogram by the square root of the height in meter. AGD measurement was conducted in the operating room or the treatment room to protect the subjects' privacy and personal information. The subjects were first placed in the supine position and changed to the lithotomy position in which the legs are spread apart to be put on rests. Then, a researcher disinfected their pudenda with Betadine solution by wearing sterile gloves to prevent infection. The length between the posterior commissure of labia (point 1) and the center of the anus (point 2) was measured in millimeter (mm) by rounding up to zero decimal places using a sterilized paper ruler (Fig. 1).
Mean value, standard deviation, and minimum value and maximum value for all variables to be measured of this study were calculated. To control height and weight, AGI was also calculated since AGD is likely to be affected by the two variables. AGI was calculated using the formula and by rounding off to the nearest hundredth.
This study used the Mann-Whitney U test to see the difference between AGD of pre-menopausal and post-menopausal women out of adult females and AGI in which height and weight were reflected. The statistical analysis was carried out using SPPS for Windows Version 18.0 (SPSS Inc., Chicago, IL, USA). If P value was less than 0.05, it was considered statistically significant.
To compare AGD of pre-menopausal women and post-menopausal women, a total of 50 subjects including 25 in each group were examined. For a comparison between two groups, their characteristics were expressed as 'mean ± standard deviation (SD; minimum value - maximum value).' Height was significantly different between two groups with 162 ± 5 cm (152 to 170) of pre-menopausal women and 151 ± 7 cm (131 to 165) of post-menopausal women. On the other hand, weight did not significantly differ between two groups with 54.9 ± 8.7 kg (42.8 to 75.1) of pre-menopausal women and 56.0 ± 8.0 kg (36.9 to 73.0) of post-menopausal women. BMI significantly varies between two groups with 20.9 ± 3.5 m/kg2 (16.9 to 29.8) of pre-menopausal women and 24.4 ± 3.1 m/kg2 (19.6 to 31.0) of post-menopausal women. Birth history that included both a vaginal delivery and a caesarean delivery was significantly different between two groups with 0.5 ± 0.7 (0 to 2) of pre-menopausal women and 3.4 ± 1.5 (0 to 6) of post-menopausal women. A vaginal delivery that may affect AGD also significantly differs between two groups with 0.3 ± 0.6 (0 to 2) and 3.2 ± 1.6 (0 to 6e),s prectively.
Mann-Whitney U Test was used to compare AGD of pre-menopausal women and post-menopausal women which is the purpose of this study. AGD distribution was 34.8 ± 6.4 cm (22 to 43) in pre-menopausal women and 30.3 ± 6.6 cm (20 to 50) in post-menopausal women (Fig. 2). The comparison of mean values between two groups showed significant difference of P = 0.019. Also since AGD may be affected by height and weight, the mean values of two groups were compared by rounding off to the nearest tenth of AGI which was calculated by dividing AGD by BMI in order to correct them. The difference between two groups was significant (P = 0.000) with 1.7 ± 0.4 (1.1 to 2.4) of pre-menopausal women and 1.3 ± 0.3 (0.8 to 2.0) of post-menopausal women (Table 1).
Mann-Whitney U Test was used to assess the relationship between AGD and birth history that was different between two groups as shown above. Most of all, the subjects were divided into the group having experience of vaginal delivery and the group having no experience of it regardless of menopause, and the mean values of AGI which were corrected by AGD and BMI were compared. The number of women having no experience of vaginal delivery was 21, and their AGD was 34.4 ± 7.1 mm and AGI was 1.7 ± 0.4. The number of women having experience of vaginal delivery at least once was 29 with 31.2 ± 6.5 mm of AGD and 1.3 ± 0.3 of AGI (Table 2). No significant difference in AGD was seen between two groups, but the difference in AGI was statistically significant. Next, the subjects were divided into the pre-menopausal group and the post-menopausal group to identify the differences in AGD and AGI according to the history of vaginal delivery. The number of pre-menopausal women having no experience of vaginal delivery was 19, and their AGD was 34.7 ± 6.8 mm and AGI was 1.7 ± 0.4. The number of pre-menopausal women having experience of vaginal delivery was 6 with 34.8 ± 5.8 mm of AGD and 1.6 ± 3.4 of AGI. No significant differences in AGD and AGI were found between two groups. The same investigation was applied to the post-menopausal group. The number of post-menopausal women having no experience of vaginal delivery was 2 and their AGD was 31.0 ± 12.7 mm and AGI was 1.5 ± 0.8. The number of post-menopausal women having experience of vaginal delivery was 23 with 30.2 ± 6.4 mm of AGD and 1.2 ± 0.3 of AGI. Consequently, no significant differences in AGD and AGI were discovered between the pre-menopausal group and the post-menopausal group according to the history of vaginal delivery (Table 3).
AGD of animals, newborn infants, and adult males have been frequently researched, but a few studies have been made on AGD of adult females. In animal tests, AGD was usually used to test toxicity. For instance, phthalates which is phthalic acid diesters is synthetic chemical compound used in cosmetics, shampoo, and soap. It affects AGD of rodents and human models to make it shorter than that of a control group.1,4 On the contrary, AGD of rodents, mammals, and newborn male infants exposed to male sex hormone at the early stage was long.5,6
In normal rodents without any disease, males' AGD was twice as long as females' AGD, and similar results were reported in humans.7 In mammals, AGD is influenced by male sex hormone. Therefore the exposure to phthalates that serves as male sex hormone antagonist at the fetal period curbs the sending of signals by acting upon male sex hormone receptors, reducing the synthesis of testosterone and hindering masculinization.4 If AGD gets shorter due to the excessive exposure to environmental hormones, abnormalities are likely to occur in the development of male reproductive system including hypoplasia of epididymis, undescended testis, and hypospadias.8
A research on AGD of adults found that AGD of men exposed to a low dose of testosterone caused by gonadal hypofunction was significantly shorter than that of men exposed to a high dose of testosterone.9 A similar study reported that short AGD is related to testicular dysgenesis syndrome.10 It found that since AGD was different among newborn infants depending on pregnancy period, AGD can be used as an anthropometric marker.11
There is lack of anatomical study as well as study on AGD of adult females. Menopause causes physical and emotional changes in women as estrogen is no longer produced in the ovary. Estrogen receptor (ER) exists in various areas of the human body and they are divided into ER1 and ER2. ER1 is present in the ovary, uterus, vagina, breast tissue, and vaginal epithelium, while ER2 is present in the ovary, testis, and hypothalamus. Estrogen acts on receptors on the skin to increase collagen production under the skin.12,13,14 The same is true of external genitalia. Post-menopausal decrease of estrogen lowers the production of collagen, resulting in atrophoderma, xeroderma, and skin lacking elasticity. It also works on female genitalia to make the skin layer of vagina and pudendum thin, constrict the epithelium, and reduce wrinkles. As a result, the overall vaginal length (from cervix to introitus) gets shorter and elasticity reduces.15 Under the hypothesis, this study intended to identify if there is a correlation between the change of external genitalia and the change of AGD following the reduced secretion of estrogen after menopause.
This study had several limitations since it was a pilot study conducted before collecting sufficient samples by reflecting the reality in which the study of AGD in adult females lacks. First, the number of samples was merely 50 including 25 each in the experimental group and the control group. The sample size was too small to represent a subject population because it was not a multicenter study. Second, there was significant difference in delivery history between two groups. This study could not completely control a variable of delivery history, which is likely to affect the result. Third, this study only dealt with pre-menopausal and post-menopausal women, so that those in menopausal transition were excluded and the change of AGD was not confirmed. Therefore if an additional multicenter cohort study that includes women in menopausal transition is conducted in the future, it is expected to reduce the study error and obtain meaningful outcomes.
Females after menopause experience various physical changes that have a great deal of influence on every part of the body such as skin elasticity, bone production, and bone resorption as well as female genitalia including the uterus, ovary, and vagina. The result of this study was that AGD of post-menopausal women was significantly shorter than that of pre-menopausal women, and it was believed to be resulted from a drop in collagen production, atrophoderma, and changes in external genitalia caused by the decreased secretion of estrogen after menopause. According to the results, AGD can be used as a metric to question the possibility of diseases related to menopause. In the future, various additional studies are needed. First, studies to enhance confidence based on a large sample size and to explore if delivery affects AGD are needed. On the other side, the study found the difference of AGI for the history of vaginal delivery as pre-menopausal and post-menopausal women were included. However when they were divided into the pre-menopausal group and the post-menopausal women and the difference for the history of vaginal delivery by group was confirmed, no statistical significance was found in two groups. Therefore it is required to conduct additional study of the correlation between vaginal delivery history and AGD in the future. This attempt may be an opportunity to develop through the relation between a atherosclerosis or osteoporosis and AGD.16,17
Figures and Tables
Fig. 1
Measurement of anogenital distance (AGD), AGD is defined by distance between the posterior commussure of labia and anus. AGD was measured in lithotomy position. AGD: anogenital distance, AGI: anogenital index, BMI: body mass index.
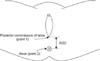
Fig. 2
Anogenital distance (AGD) and anogenital index (AGI) in pre- and postmenopausal women (n = 25 in each group).

References
1. Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, et al. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci. 2000; 58:339–349.
2. Mendiola J, Roca M, Mínguez-Alarcón L, Mira-Escolano MP, López-Espín JJ, Barrett ES, et al. Anogenital distance is related to ovarian follicular number in young Spanish women: a cross-sectional study. Environ Health. 2012; 11:90.
3. Basaran M, Kosif R, Bayar U, Civelek B. Characteristics of external genitalia in pre- and postmenopausal women. Climacteric. 2008; 11:416–421.
4. Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005; 113:1056–1061.
5. Gray LE Jr, Wilson VS, Stoker T, Lambright C, Furr J, Noriega N, et al. Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. Int J Androl. 2006; 29:96–104.
6. Barrett ES, Parlett LE, Sathyanarayana S, Liu F, Redmon JB, Wang C, et al. Prenatal exposure to stressful life events is associated with masculinized anogenital distance (AGD) in female infants. Physiol Behav. 2013; 114-115:14–20.
7. Salazar-Martinez E, Romano-Riquer P, Yanez-Marquez E, Longnecker MP, Hernandez-Avila M. Anogenital distance in human male and female newborns: a descriptive, cross-sectional study. Environ Health. 2004; 3:8.
8. Wilson VS, Blystone CR, Hotchkiss AK, Rider CV, Gray LE Jr. Diverse mechanisms of anti-androgen action: impact on male rat reproductive tract development. Int J Androl. 2008; 31:178–187.
9. Mendiola J, Stahlhut RW, Jorgensen N, Liu F, Swan SH. Shorter anogenital distance predicts poorer semen quality in young men in Rochester, New York. Environ Health Perspect. 2011; 119:958–963.
10. Eisenberg ML, Jensen TK, Walters RC, Skakkebaek NE, Lipshultz LI. The relationship between anogenital distance and reproductive hormone levels in adult men. J Urol. 2012; 187:594–598.
11. Kim TH, Lee HH, Kim JM, Yang YJ, Kim SY, Hong YP. The routine value of anogenital distance as an anthropometric measurement in newborns. Clin Exp Obstet Gynecol. 2014; 41:283–287.
12. Hall G, Phillips TJ. Estrogen and skin: the effects of estrogen, menopause, and hormone replacement therapy on the skin. J Am Acad Dermatol. 2005; 53:555–568.
13. Castelo-Branco C, Duran M, González-Merlo J. Skin collagen changes related to age and hormone replacement therapy. Maturitas. 1992; 15:113–119.
14. Mun MJ, Kim JH, Kim TH, Hwang JY, Jang WC. Associations between estrogen receptor gene polymorphisms and endometriosis. J Korean Soc Menopause. 2013; 19:64–73.
15. Brincat M, Kabalan S, Studd JW, Moniz CF, de Trafford J, Montgomery J. A study of the decrease of skin collagen content, skin thickness, and bone mass in the postmenopausal woman. Obstet Gynecol. 1987; 70:840–845.
16. Barengolts EI, Berman M, Kukreja SC, Kouznetsova T, Lin C, Chomka EV. Osteoporosis and coronary atherosclerosis in asymptomatic postmenopausal women. Calcif Tissue Int. 1998; 62:209–213.
17. Mitchell BD, Kammerer CM, Schneider JL, Perez R, Bauer RL. Genetic and environmental determinants of bone mineral density in Mexican Americans: results from the San Antonio Family Osteoporosis Study. Bone. 2003; 33:839–846.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download