Abstract
Sclerosing stromal tumor (SST) of the ovary is a rare tumor derived from the sex cord stroma. This tumor was first described by Chalvaridjian and Scully in 1973. SST of the ovary is prevalence of 1.5% to 6% of ovarian stromal tumors. Patients are most commonly diagnosed in their 20s and 30s. There have been reports of SST postmenopausal women aged 65-, 67-, and 71 in the Republic of Korea; however, no report of this disease has been reported in women older than 80. In this study, we would like to report an 80-year-old postmenopausal woman who did not previously complain of any symptoms, and was finally diagnosed with SST. She was involved in a traffic accident, and huge pelvic mass was found during the evaluation of intra-abdominal hemorrhage. Total abdominal hysterectomy with bilateral salpingo-oophorectomy was performed ; a final pathologic diagnosis reported SST.
Age: 80-years old woman.
Chief complaint and present illness: Rib fractures and hemoperitoneum due to traffic accident. After having general surgery and orthopedic surgery, she was transferred to gynecology department to evaluate a huge pelvic mass found by transabdominal ultrasonography by chance.
Obstetric history: T3-P0-A0-L3
Menstrual history: Menarche at 12 years old, regular period by 28 days, no severe menstrual pain, menopause at 50 years old.
Past history: No particular medical history.
Laboratory findings (at admission): Hemoglobin 9.7 g/dL, hematocrit 28.3%, white blood cell 8,160/mm3, platelet 372,000/mm3. There was no specific finding in liver function test, kidney function test, coagulation test, urinalysis, echocardiogram, or chest X-ray. Antibody for hepatitis B virus or syphilis was negative. Tumor markers were as followed; alpha-fetoprotein (αFP) 11.6 ng/mL (normal range 0-7.5), carcinoembryonic antigen (CEA) 2.28 ng/mL (normal range 0-4.7), carbohydrate antigen 19-9 (CA19-9) 6.8 U/mL (normal range 0-27), cancer antigen 125 (CA-125) 114.8 U/mL (normal range 0-35.0), β-human chorionic gonadotropin (β-HCG) 1.8 mIU/mL, squamous cell CA (SCCA) 2.50 ng/mL (normal range 0.1-1.5).
Pelvic ultrasonographic findings: Huge mass (12 cm × 8.5 cm) was found in pelvic cavity.
Magnetic Resonance Imaging (MRI) findings: A mass as large as 9 cm × 10.5 cm × 10 cm was found in the pelvic cavity. Most of the part showed low signal intensity, while several irregular parts of it exhibited high signal intensity (Fig. 1). Mass was much less enhanced than the myometrium around it. Large part of the mass adjoined the uterus, but there was no artery branching from the uterine artery to feed the mass. Instead, an artery from the right adnexa gave blood supply to the mass. Therefore, it seemed more possible that the mass had originated from ovarian fibroma, or extrauterine leiomyoma of right adnexa. A polypoid mass about one centimeter was located posterior to the uterus, and also had small part of lower signal intensity within the mass. This one was not enhanced, which could be a sign of hemorrhaging. Ovaries were atrophied, and there was pelvic fluid collection in the pelvic cavity.
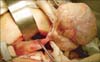
Operative findings: We started exploratory laparotomy with an impression of ovarian cancer, and total hysterectomy and bilateral salpingo-oophorectomy was done as a result. Small amount of ascites was seen in peritoneal cavity. A mass of 11 cm diameter originating from the right ovary was confirmed (Fig. 2), and the uterus seemed slightly enlarged. There was no palpable lymph node or nodule in the peritoneal cavity.
Sclerosing stromal tumor (SST) was first reported by Chalvaridjian and Scully1 in 1973. SST of the ovary is an extremely rare benign ovarian tumor of which the prevalence is from 1.5% to 6%, and occurs frequently in the second and third decades.1,2 Patients who have this kind of tumor may have irregular menstruations, menorrhagia, or lower abdominal pain. Therefore, when a clinician see young women express any of these symptoms, clinicians may think of SST as one of the differential diagnosis.3 However, it is far more difficult to diagnose SST in elderly women for many reasons; the incidence is much lower in elderly women, symptoms related to menstruation cycle can be hidden due to patients' menopause, and various common conditions can cause non-specific abdominal pain in senior populations. Final diagnosis of SST is usually confirmed after pathologic examination after surgery. SSTs usually have portions with higher cellularity, stromal portion dominantly with collagen, and edematous portion, so that pathologists can distinguish it from fibroma, thecoma, or lipoid cell tumors.4
Most of the SSTs are non-functioning tumors, which do not manipulate endocrinal function.5 SSTs are usually found in the second to third decades, not like other kinds of stromal tumors of which the prevalence is high in the fifth or sixth decades. It is usually in the solid shape which is well demarcated from surrounding tissue.6,7 Histologically, there is no hyaline degeneration which is quite common in fibroma or thecoma, angioproliferative, and pseudolobulation due to focal edema.8 It shows histological difference with sex-cord stromal tumor by rare epithelial component and high in mesenchymal component.8 Patients usually complain irregular menstruation, hypermenorrhea, or abdominal or pelvic pain, but still non-symptomatic patients exist even before menopause. Postmenopausal women generally do not experience any symptoms like this case, however there have been several cases with prolonged vaginal bleeding after menopause or cystic endometrial hyperplasia.9 It is very rare to be found in both ovaries and right ovary is more susceptible to this tumor, showing 71% of cases to be found on right ovary.10 Chalvardjian and Scully1 studies ten patients who showed abnormal menstrual symptoms and dysfunctional uterine bleeding but with no evidence of hormonal imbalance. However, Damajanov11 reported steroidal hormonal imbalance in SST patients, and normalization of urinary secretion of estrogen and androgen after resection of the SST. Some cases showed elevation of CA-125, but SSTs are considered to be benign tumors which can be cured by oophorectomy or ovarian cystectomy, and recurrence or metastasis have not been reported.10 SSTs are rare diseases; therefore in most of the cases, no hormonal evaluation is done before surgery. Differential diagnosis of malignant tumors is necessary, especially in elderly patients since SSTs are rare in senior patients. Ascites is very rare in this tumor.9 There is no certain pathognomonic ultrasonographic findings. T2 weighted image of MRI shows high signal of stromal part and scattered small nodule of low signal, which is pathognomonic image of pseudolobulation.8 Severe ovarian edema or Krukenberg tumor should be excluded pathologically by nuclear dysplasia.
There have been ten cases of SST reported in Republic of Korea. However, it is rare to find SST in eighties, and it is the first case in Republic of Korea.3 SST is very hard to differentiate from other malignant tumors, so histological study must be done to diagnose SST in senior patients.12 This case shows a pelvic mass found in elderly patients could be a benign tumor such as SST, even when it seems malignant in imaging studies.
Figures and Tables
Acknowledgement
This work was supported in part by the Soonchunhyang University Research Fund. Also, thank to librarian, Eun-Ae Jung for searching for information of literature and manuscript editing.
References
1. Chalvardjian A, Scully RE. Sclerosing stromal tumors of the ovary. Cancer. 1973; 31:664–670.
2. Iravanloo G, Nozarian Z, Sarrafpour B, Motahhary P. Sclerosing stromal tumor of the ovary. Arch Iran Med. 2008; 11:561–562.
3. Youm HS, Cha DS, Han KH, Park EY, Hyon NN, Chong Y. A case of huge sclerosing stromal tumor of the ovary weighing 10 kg in a 71-year-old postmenopausal woman. J Gynecol Oncol. 2008; 19:270–274.
4. Peng HH, Chang TC, Hsueh S. Sclerosing stromal tumor of ovary. Chang Gung Med J. 2003; 26:444–448.
5. Ho Yuen B, Robertson DI, Clement PB, Mincey EK. Sclerosing stromal tumor of the ovary. Obstet Gynecol. 1982; 60:252–256.
6. Matsubayashi R, Matsuo Y, Doi J, Kudo S, Matsuguchi K, Sugimori H. Sclerosing stromal tumor of the ovary: radiologic findings. Eur Radiol. 1999; 9:1335–1338.
7. Chang YW, Hong SS, Jeen YM, Kim MK, Suh ES. Bilateral sclerosing stromal tumor of the ovary in a premenarchal girl. Pediatr Radiol. 2009; 39:731–734.
8. Shin CS, Kim JJ, Yun CS, Cho HJ, Bae KH, Han KS, et al. A case of sclerosing stromal tumor of the ovary. Korean J Obstet Gynecol. 2003; 46:1818–1822.
9. Tang MY, Liu TH. Ovarian sclerosing stromal tumors. Clinicopathologic study of 10 cases. Chin Med J (Engl). 1982; 95:186–190.
10. Marelli G, Carinelli S, Mariani A, Frigerio L, Ferrari A. Sclerosing stromal tumor of the ovary. Report of eight cases and review of the literature. Eur J Obstet Gynecol Reprod Biol. 1998; 76:85–89.
11. Damajanov I, Drobnjak P, Grizelj V, Longhino N. Sclerosing stromal tumor of the ovary: A hormonal and ultrastructural analysis. Obstet Gynecol. 1975; 45:675–679.
12. Joja I, Okuno K, Tsunoda M, Takeda Y, Sugita K, Mizutani Y, et al. Sclerosing stromal tumor of the ovary: US, MR, and dynamic MR findings. J Comput Assist Tomogr. 2001; 25:201–206.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


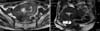

 XML Download
XML Download