Abstract
Objectives
To access the effectiveness of radiofrequency myolysis (RFM) in women with midline dysmenorrhea.
Methods
We designed RFM in two ways laparoscopic RFM (LRFM), vaginal ultrasound-guided RFM (URFM). One hundred and thirty-two patients were in the LRFM group and, 140 patients were in the URFM group.
Results
Upon receipt of surgery, both the LRFM and the URFM groups demonstrated a significant decrease (P < 0.001) in the mean pain score when compared to those before and after surgery.
Conclusion
The RF uterine myolysis procedure provides an alternative for those patients who suffer from intractable midline dysmenorrhea. LRFM is an alternative choice because it is relatively safe and, simple to perform and moreover, it is satisfactory. LRFM appears to increasingly succeed in the treatment of midline dysmenorrhea.
Dysmenorrhea, painful menstrual cramps of uterine origin, is one of the most common gynecologic complaints, ranging between 43% and 90%.1,2 Since the 1960s, medical therapy with nonsteroidal anti-inflammatory drugs (NSAIDs) and oral contraceptives with the addition of danazol and gonadotropin-releasing hormone (GnRH) has been treatment of choice for chronic pelvic pain and dysmenorrheal.3
However, rapid regrowth of the myomas to their original size has been reported to cause the recurrence of symptoms within a few months after the discontinuation of hormone treatment.4
Furthermore, GnRH agonists can obliterate the myomamyometrial interface and as a result enucleation of myomas becomes more difficult.5 Hormone therapy has been limited to premenopausal use only.
Medical treatments can be effective in some patients, there is still a 20 to 25% failure rate, in which case surgery becomes a treatment option.6
Therefore, surgical intervention has been considered a good method to treat patients who have intractable dysmenorrhea and pelvic pain. Also, it is considered the minimally invasive method of treatment for premenopausal women of severe dysmenorrhea.
Laparoscopic uterine nerve ablation (LUNA) and laparoscopic presacral neurectomy (LPSN) are two surgical interventions that have been widely used to relieve intractable dysmenorrheal.7
LPSN was shown to be more effective than LUNA in terms of long-term postsurgery pain reduction after 12 months. The presacral area is abundant in blood vessels and variables in nerve anatomy, complications could happen during and after surgery (LPSN). For these reasons surgery were performed only by experienced operators. We were trying to figure out the more effective and simple procedure than LPSN treating patients with intractable dysmenorrhea. In this study, radiofrequency myolysis (RFM) was performed on patients with midline dysmenorrhea.
We designed RFM in two ways laparoscopic RFM (LRFM), vaginal ultrasound-guided RFM (URFM).
The aim of the present study was to access the effectiveness of LRFM and URFM in patients with midline dysmenorrhea.
The study was conducted in the Department of Obstetrics and Gynecology at Chosun University Hospital. From July 2008 to July 2013, 272 reproductive female patients ranging from 32 to 47 years old who suffered midline dysmenorrhea were enrolled in a non-randomized prospective study. One hundred and thirty-two patients were in the LRFM group, 140 patients were in the URFM group.
Two hundred and seventy-two patients who had undergone surgery were prospectively surveyed regarding dysmenorrhea before and 1 year after RFM.
Informed consent was obtained. All patients were informed the risks and benefits of laparoscopic RFM. Before RFM as well as 1 year after surgery, they all completed a questionnaire that requested information on the severity of dysmenorrhea on a 0 to 4 pain score, which was similar to that devised by Chen.8,9
Severity of dysmenorrhea in all patients was scored in a 5-point scale ranging from 0 to 4 (i.e., 0 = no pain, 1 = mild pain requiring no medication, 2 = moderate pain responding to mild pain relievers, 3 = severe pain necessitating potent pain relievers, and 4 = incapacitating pain unresponsive to potent pain relievers).
We didn't administer GnRH analogs to the patients in the study. And we treated women whom they don't want to have a baby anymore; because of not being proven the relationship between RFM, with pregnancy and side effects. All examinations and procedures were performed by one gynecologist.
LRFM of uterine myomas was performed under local anes thesia using diazepam (20 mg) and pethidine (100 mg) intravenously. Prophylactic antibiotics were not used. In the lithotomy position, a uterine manipulator was inserted for better exposure of the myomas puncture site to be treated. Then, a 2 mm trocar was inserted through an umbilical incision after injection of lidocaine. The RF needle was inserted percutaneously after insufflating CO2 gas into the pelvic cavity, and placed within the target myoma under laparoscopic video guidance. The depth of the needle insertion was determined on the basis of a pre-operative ultrasound. The tip of the central prong was placed about 1-4 cm beyond the center of the myoma by size, so that the peripheral electrodes were localized where the cross-sectional area of the myoma was largest.
The target temperature for RFM was 80℃. A RF generator (RF Medical, Seoul, Korea) automatically adjusts the power to maintain the selected temperature. The electric power was fixed at 50 watts. All operations were performed by experienced one surgeons. LRFM group, a video 2 mm laparoscope was inserted via a troca placed through a subumbilical incision. Then, RF needle were inserted to uterine lesion. In our research, we filled the pelvic cavity with Adept® (Baxter, Vienna, Austria) and sprayed using a 16 G spinal needle on the region for preventing adhesions after RFM. The laparoscopy equipment was made by Karl Stortz GmbH & Co (Tuttlingen, Germany).
URFM of uterine myomas was performed under local anesthesia using diazepam (20 mg) and pethidine (100 mg) intravenously. In the lithotomy position, URFM group underwent transvaginal insertion of an ultrasonic needle into the center of the myoma(s) or lesion before myolysis. The diagnostic ultrasound system was made by Toshiba Nemio (SSA-550A; Toshiba Medical Systems Corp., Tokyo, Japan).
In this study, the Student®s t test, the Mann-Whitney U test and chi-square test were employed to compare age, operation time, length of hospitalizaton and post-operative dysmenorrhea pain score whenever appropriate. The Wilcoxon signed rank test was used to compare the difference between the pre and post-operative dysmenorrhea pain scores. The value of P < 0.05 indicated statistical significance. Statistical analysis was performed with SPSS version 12.0 (SPSS Inc., Chicago, IL, USA).
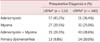
We designed a prospective study and used a zero-to-4 pain score system to evaluate 272 patients before and after RF myolysis. The classification of patients are shown in Table 1 for the LRFM and the URFM groups.
Table 2 shows no significant difference on the hospitalization length and post operative pain score between two groups. The mean length of hospital stay (± standard deviation [SD]) was 1.34 ± 0.27, 1.53 ± 0.85, respectively (P < 0.808). Most women were discharged on the day after RFM.
The pain scale scores before and after RF myolysis are reported in Table 3. The mean (± SD) post-operative pain score for dysmenorrhea was 0.83 ± 0.53 (URFM), 0.32 ± 0.78 (LRFM). In all groups, a significant difference was found in terms of pain scores for dysmenorrheal (P < 0.001) before and after RFM. No patient reported a change in the postoperative pain score during follow-up. But the mean operation time was shorter in the URFM group, compared to the LRFM group.
The comparison of complications on both groups are expressed in Table 4. No major complications occurred during or immediately following the operation. Only the increased vaginal discharge and abdominal pain were statistically different in the two groups (P = 0.006, P = 0.000).
Dysmenorrhea is a common gynecologic complaint because lasts until menopause. Previous studies have revealed that presacral neurectomy (PSN) provides an effective and relatively safe treatment for women with midline dysmenorrheal.10,11,12
An often-ignored procedure is interruption of uterosacral nerves. It was first described by Ruggi13 in 1899, since then European gynecologists have performed it frequently. In 1955, vaginal transaction of uterosacral nerves provided relief to over 70% of patients.14 These results were similar to those tabulated for PSN. The surgical approach of incorporating interruption of the superior hypogastric nerve plexus (PSN) is less popular.8
Laparoscopic surgery has become increasingly popular in recent years and plays an important role in treating patients with dysmenorrheal.15
So, LPSN was shown to be more effective than LPSN in terms of long-term postsurgery pain reduction for midline dysmenorrhea after 12 months.
But the presacral area is abundant in blood vessels and variables in nerve anatomy, complications could happen during and after LPSN.
Several new techniques for myolysis have been sought in an attempt to achieve minimally invasive approaches to uterine myomas which are less technically demanding, and less time consuming than laparoscopic myomectomy, while minimizing adhesion formation.16
Even though laparoscopic or open myomectomy are considered the classic surgical approaches for women who desire uterine preservation, the current literature on this subject underlines the need for new, alternative, less invasive techniques to treat symptomatic myomas. Myolysis as a treatment option for uterine myomas was first introduced in the late 1980s as a hysteroscopic technique.17 Subsequently, myolysis was performed as a variation on the technique of laparoscopic myomectomy in which myomas are coagulated rather than removed.
The RF uterine myolysis procedure provides an alternative for those patients who suffer intractable midline dysmenorrheal. This procedure is relatively simple and safe and could result in a satisfactory outcome in the intervention of midline dysmenorrheal.
We designed a prospective study and used a zero-to-4 pain score system to evaluate 272 patients before and after RFM. No patient had intraoperative or long-term complications.
We conclude that dysmenorrheal statistically significantly improved after RFM in women with symptomatic uterine myomas.
Both the LRFM and URFM effectively reduced midline dysmenorrhea caused by myoma and adenomyosis. RF energy desiccate target tissue directly of disrupt their blood supply and destroy pain receptors in nerve fibers in the uterus. RFM markedly reduced the midline component of menstrual pain.
Therefore LRFM may provide a better operative field and complete resection in comparison to URFM. LRFM is an effective treatment for severe central pelvic pain and intractable dysmenorrhea. It is safe and effective for treating midline dysmenorrheal and pelvic pain.
When a patient requires surgical treatment for chronic midline pelvic pain or severe dysmenorrhea, LRFM is an al ternative choice. It is relatively safe, simple to perform and satisfactory. LRFM is an alternative choice because it is relatively safe and, simple to perform and moreover, it is satisfactory. A larger series of patients and precise methodology, including a more detailed pain score system of inclusion criteria, nonetheless, are necessary to fully evaluate the efficacy of the LRFM procedure.
Figures and Tables
References
1. Jamieson DJ, Steege JF. The prevalence of dysmenorrhea, dyspareunia, pelvic pain, and irritable bowel syndrome in primary care practices. Obstet Gynecol. 1996; 87:55–58.
2. Hurd WW. Criteria that indicate endometriosis is the cause of chronic pelvic pain. Obstet Gynecol. 1998; 92:1029–1032.
3. Chang Y, Tsai EM, Long CY, Lin WC. A modified method of laparoscopic presacral neurectomy for the treatment of midline dysmenorrhea. J Minim Invasive Gynecol. 2006; 13:211–215.
4. Liu WM, Ng HT, Wu YC, Yen YK, Yuan CC. Laparoscopic bipolar coagulation of uterine vessels: a new method for treating symptomatic fibroids. Fertil Steril. 2001; 75:417–422.
5. Campo S, Garcea N. Laparoscopic myomectomy in premenopausal women with and without preoperative treatment using gonadotrophin-releasing hormone analogues. Hum Reprod. 1999; 14:44–48.
6. Henzl MR. Dysmenorrhea: achievements and challenge. Sex Med Today. 1985; 9:8–12.
7. Chang CY, Chang WC, Hung YC, Ho M, Yeh LS, Lin WC. Comparison of a new modified laparoscopic presacral neurectomy and conventional laparoscopic presacral neurectomy in the treatment of midline dysmenorrhea. Int J Gynaecol Obstet. 2007; 99:28–32.
8. Chen FP, Chang SD, Chu KK, Soong YK. Comparison of laparoscopic presacral neurectomy and laparoscopic uterine nerve ablation for primary dysmenorrhea. J Reprod Med. 1996; 41:463–466.
9. Chen FP, Soong YK. The efficacy and complications of laparoscopic presacral neurectomy in pelvic pain. Obstet Gynecol. 1997; 90:974–977.
10. Proctor ML, Latthe PM, Farquhar CM, Khan KS, Johnson NP. Surgical interruption of pelvic nerve pathways for primary and secondary dysmenorrhoea. Cochrane Database Syst Rev. 2005; CD001896.
11. Perez JJ. Laparoscopic presacral neurectomy. Results of the first 25 cases. J Reprod Med. 1990; 35:625–630.
12. Carter JE. Laparoscopic treatment for chronic pelvic pain: results from three-year follow-up. J Am Assoc Gynecol Laparosc. 1994; 1:S6–S7.
13. Ruggi J, Crossen HS, editors. Gynecology. St Louis, MO: CV Mosby;1915.
14. Doyle JB. Paracervical uterine denervation by transection of the cervical plexus for the relief of dysmenorrhea. Am J Obstet Gynecol. 1955; 70:1–16.
15. Gambone JC, Mittman BS, Munro MG, Scialli AR, Winkel CA. Consensus statement for the management of chronic pelvic pain and endometriosis: proceedings of an expert-panel consensus process. Fertil Steril. 2002; 78:961–972.
16. Bergamini V, Ghezzi F, Cromi A, Bellini G, Zanconato G, Scarperi S, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005; 192:768–773.
17. Donnez J, Gillerot S, Bourgonjon D, Clerckx F, Nisolle M. Neodymium: YAG laser hysteroscopy in large submucous fibroids. Fertil Steril. 1990; 54:999–1003.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download