Abstract
Mayer-Rokitansky-Kuster-Hauser syndrome (MRKHS) is characterized by vaginal agenesis with variable Müllerian duct abnormalities. We report here a case of uterine adenomyosis which developed from a hypoplastic uterus in a patient with MRKHS. A 55-year-old postmenopausal woman visited a university hospital for pelvic mass. She had underwent vaginoplasty via the McIndoe procedure for MRKHS at 15 years of age. Pelvic magnetic resonance imaging showed a 5.4 × 4.8 × 4.7 cm mass suspicious for a uterine myoma. She received total abdominal hysterectomy with bilateral salpingo-oophorectomy, and neither the cervix nor endometrium was found grossly in the surgical specimen. The final histologic diagnosis was uterine adenomyosis.
Mayer-Rokitansky-Kuster-Hauser syndrome (MRKHS) or Müllerian agenesis, is characterized by vaginal agenesis with variable Müllerian duct abnormalities. It is a relatively common cause of primary amenorrhea, and the incidence is approximately 1 in 5,000 newborn girls in Finland.1,2 They exhibit normal, symmetrical breast and pubic hair development, no visible vagina, and have no symptoms or signs of crytomenorrhea because the rudimentary uteri contain no functional endometrium, but, in approximately 10%, functional islands of endometrium may result in a hematometra and symptoms of cyclic pain.1,3 Magnetic resonance imaging (MRI) is still the most standardized tool for diagnosis, but three-dimensional computed tomography and a laparoscopic approach may be a feasible choice of diagnosis.4 Traditional operative treatment of women with Müllerian agenesis is McIndoe procedure, which is surgical creation of a neovagina involves dissection of the rectovaginal space and placement of a skin graft, held in place with a soft mold until the graft becomes established.5
Uterine adenomyosis is a benign disorder characterized by the extension of endometrial glands and stroma into the myometrium, and it is a clinical diagnosis.1,6 Diffuse uterine enlargement is common in patients with uterine adenomyosis, but focal nodular lesions called adenomyomas develop in some women, which clinically resemble leiomyomas.
We report here a case of uterine adenomyosis which developed from hypoplastic uterus in postmenopausal women who had previously underwent McIndoe procedure for MRKHS.
A 55-year-old woman with a previous history of McIndoe operation for MRKHS at 15 years of age was referred to the gynecological clinic at Inje University Haeundae Paik Hospital following the diagnosis of a pelvic mass during an opportunistic health check.
Gynecologic examination revealed a hen's egg sized mass in the pelvic cavity, possibly the uterus, and also revealed the absence of uterine cervix. External genitalia appeared grossly normal. Laboratory findings showed elevated gonadotropin levels (luteinizing hormone [LH], 61.50 mIU/mL; follicle stimulating hormone [FSH], 94.80 mIU/mL) and a normal cancer antigen 125 (CA 125) level (9.25 U/mL).
Pelvis MRI showed a 5.4 × 4.8 × 4.7 cm mass suspicious to be uterine myoma, and also demonstrated that the mass was not connected to the vaginal cavity (Fig. 1A, 1B).
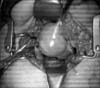
Laparotomy revealed a 5 cm sized uterine mass occupying the lower pelvic cavity, and both adnexae were small and atrophied (Fig. 2). Uterine cervix could not be palpated definitely. She underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy.
The multinodular and abnormal shaped uterus was 7.0 × 6.2 × 4.5 cm in size (Fig. 3A), and the endometrium, cervix and vagina were not clearly identified in the cut surface (Fig. 3B). The ovary and salpinx were grossly normal shaped but atrophied (Fig. 3C, white arrows). Microscopically, the whole uterus showed adenomyosis features, such as multifocal atrophic endometrial glands and stroma in the background of proliferating smooth muscle cell bundles (Fig. 4A). The atrophic endometrium (< 1 mm thickness) was seen rarely in the lower segment of the uterus. (Fig. 4B). However, there is no evidence of a cervix or vagina in the lower segment of the uterus. No abnormalities were recognized in either the ovaries or the salpinges.
This is the first case report of uterine adenomyosis which developed from hypoplastic uterus in postmenopausal woman with MRKHS in Korea. To our knowledge, there were only two cases of adenomyosis in a patient with MRKHS reported.7,8 Enatsu et al.7 previously reported a case about adenomyosis in a patient with MRKHS. A 27-year-old Japanese woman who had received vaginoplasty by using the McIndoe procedure for MRKHS at age 19 came to university hospital for evaluation of severe lower left abdominal pain that occurred every month. MRI showed a tumor 5 cm in diameter in lower left abdomen, with irregular intensity and no myoma nodules. She had received laparoscopic tumor resection, and histologic examination revealed adenomyosis. The other reported case was about a 52-year-old Chinese woman with MRKHS who underwent hysterectomy and bilateral salpingo-oophorectomy for painful uterine mass and were diagnosed with uterine fibroids and adenomyosis.8
The established view is that uterine adenomyosis always represents a downgrowth from the basal layer of the endometrium,8 which means that adenomyosis arise through direct invasion of the uterine mucosa into the uterine musculature. But, this established concept is hard to explain how adenomyosis develop in Müllerian remnants in patient with MRKHS. Enatsu et al.7 reported that there existed endometrium-like tissues in myometrium of their patient who did not have functional endometrium. In our case, there were not found definite communication between adenomyosis in myometrium and atrophic endometrium, either. Therefore, the histogenesis of adenomyosis in our case may not be a mechanism of direct invasion, but be a result of metaplasia of Müllerian remnants inside the hypoplastic uterus, which is consistent with the proposal by Nisolle and Donnez9 that histopathogenesis of the rectovaginal endometriosis nodule is not related to transplantation by retrograde menstruation but related to metaplasia of Müllerian remnants in the rectovaginal septum.7,9 Chun et al.10 reported a case of simple endometrial hyperplasia of ectopic endometrial tissue in myometrium with normal endometrial cavity in patient with adenomyosis, and the authors proposed the possibility of spontaneous hyperplasia of ectopic endometrium independent of eutopic endometrium, which partially supports the hypothesis of metaplasia in the development of adenomyosis. Further researches are needed to determine whether uterine adenomyosis could develop by metaplasia besides direct invasion of eutopic endometrium.
Figures and Tables
Fig. 1
Pelvis magnetic resonance imaging. (A) Uterine mass, coronal view. (B) Uterine mass, sagittal view.
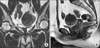
Fig. 3
Gross findings of the specimen. Multinodular uterus shows adenomyoma or myoma like features and the atrophic endometrum as well as the aplastic uterine cervix and vagina. White arrow points to the atrophic ovary and salpinx.
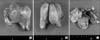
Fig. 4
Histologic findings from the resected specimen. (A) Uterine myometrium (H&E, × 200). Microscopically, the uterus shows typical adenomyosis features, such as multifocal atrophic endometrial glands and stroma in the background of proliferating smooth muscle cell bundles. (B) Uterine endometrium (H&E, × 100). The atrophoic endometrium (< 1 mm thickness) is seen in the lower segment of the uterus.

References
1. Fritz MA, Speroff L, editors. Clinical gynecologic endocrinology and infertility. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins;2010. p. 455–457. p. 612–613.
2. Aittomäki K, Eroila H, Kajanoja P. A population-based study of the incidence of Mullerian aplasia in Finland. Fertil Steril. 2001; 76:624–625.
3. Fedele L, Bianchi S, Frontino G, Ciappina N, Fontana E, Borruto F. Laparoscopic findings and pelvic anatomy in Mayer-Rokitansky-Kuster-Hauser syndrome. Obstet Gynecol. 2007; 109:1111–1115.
4. Kim TH, Lee HH, Jeon DS, Park J. Laparoscopic resection of the rudimentary horn of a unicornuate uterus diagnosed by three-dimensional computed tomography. Med Case Stud. 2013; 4:9–12.
5. McIndoe AH, Banister JB. An operation for the cure of congenital absence of the vagina. BJOG. 1938; 45:490–494.
6. Rapkin AJ, Howe CN. Pelvic pain and dysmenorrhea. In : Berek JS, Novak E, editors. Berek & Novak's gynecology. 14th ed. Philadelphia, PA: Lippincott Williams & Wilkins;2007. p. 505–540.
7. Enatsu A, Harada T, Yoshida S, Iwabe T, Terakawa N. Adenomyosis in a patient with the Rokitansky-Kuster-Hauser syndrome. Fertil Steril. 2000; 73:862–863.
8. Yan CM, Mok KM. Uterine fibroids and adenomyosis in a woman with Rokitansky-Kuster-Hauser syndrome. J Obstet Gynaecol. 2002; 22:561–562.
9. Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997; 68:585–596.
10. Chun S, Jeon GH, Cho HJ, Kim YM, Ji YI. Endometrial hyperplasia in myometrium of woman with uterine adenomyosis: a case report. J Reprod Endocrinol. 2012; 4:56–60.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download