Abstract
Background
Cell adhesion molecules (CAMs) expressed on hematopoietic progenitor cells (HPCs), endothelial cells, and stromal cells play a pivotal role in the mobilization of CD34+ cells. Herein, we conducted a non-randomized peripheral blood stem cell (PBSC) mobilization study aimed to compare the potential differences in the expressions of several CAMs and chemokines on CD34+ cells obtained from bone marrow aspirate before and after HPC mobilization from patients with hematologic malignancies and healthy donors.
Methods
Three-color cytofluorometric analysis was used to compare the expressions of CAMs and chemokines in the bone marrow before and after mobilization.
Results
For all studied groups, CAM expression among those with good and poor yields of CD34+ cells was significantly correlated with VCAM-1 (P=0.007), CD44 (P=0.027), and VLA-4 (P=0.014) expressions. VCAM-1 (P=0.001), FLT-3 (P=0.001), CD44 (P=0.011), VLA-4 (P=0.001), and LFA-1 (P=0.001) expressions were higher before HPC mobilization than after HPC mobilization. By contrast, the expression of CXCR4 significantly varied before and after mobilization only among those with successful PBSC mobilization (P=0.002).
Conclusion
We attempted to identify particular aspects of CAMs involved in CD34+ cell mobilization, which is a highly complex mechanism that involves adhesion molecules and matrix metalloproteases. The mechanism by which CD34+ cell mobilization is activated through proteolytic enzymes is not fully understood. We believe that CXCR4, VLA-4, CD44, and VCAM-1 are the most important molecules implicated in HPC mobilization, particularly because they show a correlation with the yield of CD34+ cells collected via large volume leukapheresis.
Peripheral blood stem cells (PBSC) have become the main source for autologous and allogeneic marrow transplantation because they are associated with earlier neutrophil and platelet engraftment and shorter hospitalization than bone marrow (BM) stem cells [12]. A substantial amount of CD34+ cells can be mobilized from the BM into the peripheral blood (PB) by hematopoietic growth factors alone or in combination with cytotoxic agents and the partial CXC chemokine receptor-4 antagonist, plerixafor [234]. The prerequisite for autologous stem cell transplantation (ASCT) is successful and adequate stem cell mobilization and collection. Depending on the criteria, failure rates range from 5% to 40% [45]. Despite the high success rate of PBSC trans plantation, the specific mechanisms involved in PBSC mobilization and homing are unclear [4]. The efficacy of mobilization is related to several factors, including the number of prior therapies and the mobilization protocols [246]. The number of CD34+ cells in the PB before PBSC collection is correlated with the yield of CD34+ cells in the apheresis product (AP) and it is used to determine when to begin collection [123]. However, specific tools that can be applied before initiating mobilization regimens to predict the yield of CD34+ cells are still unavailable. Adhesive interactions between the CD34+ hematopoietic progenitor cells (HPCs) and the cellular and matrix components of the BM environment are involved in the mobilization [789]. Under steady-state conditions, HPCs are mainly in close contact with the BM microenvironment. Various cell adhesion molecules (CAMs) and chemokines are expressed on HPCs, including β1 integrins, such as very late antigen-4 (VLA-4) (CD49d/CD29) and VLA-5 (CD49e/CD29); β2 integrins, such as leukocyte function antigen-1 (LFA-1) (CD11a/CD18); selectins, such as L-selectin (CD62L); and members of the immunoglobulin super-family, such as intercellular adhesion molecule-1 (ICAM-1) (CD54), vascular cell adhesion molecule-1 (VCAM-1) (CD106), and the chemokine CXCR4 receptor of stromal-derived factor-1 (SDF-1) and CD44, the major receptor of hyaluronic acid (HA) [789101112131415]. The interaction between VCAM-1, which is expressed in BM stromal, and the counter-receptor integrin VLA-4, expressed at the surface of HPCs, is critical to the homing and release of HPCs in the BM [1112]. A second pathway implicated in the trafficking of HPCs is the CXCR4/SDF-1 chemotactic axis [1314]. Plerixafor selectively disrupts the adhesion between CXCR4 on CD34+ cells and its ligand CXCL12, which is expressed by marrow stromal cells, thereby causing the release of marrow CD34+ cells into peripheral circulation [5131415].
CD44 is important in cell migration in various normal and malignant cells. CD44 is a multifunctional and multistructural receptor with numerous isoforms. Standard CD44 (CD44s), the smallest CD44 molecule, which lacks the entire variable region, is the most common isoform expressed on HPCs. The major ligand of CD44 is HA, an important component of the extracellular matrix in many different organs, including the BM where it is produced by both stromal and hematopoietic cells. During granulocyte colony stimulating factor (G-CSF)-mediated mobilization, neutrophil degranulation occurs, leading to upregulation of the matrix metalloproteases, which in turn causes cleavage of CD44 and a decrease in CD44 expression [1617].
FLT3 is a type III tyrosine kinase receptor expressed mainly by primitive hematopoietic cells. FLT3L, the ligand for this receptor, is also a transmembrane protein expressed on various cells, including fibroblasts and T lymphocytes [18]. FLT3L is a stem-cell specific growth factor that expands and may also mobilize stem cells in mice after its administration for 10 days either as a single agent or in combination with other molecules, such as interleukin 8 (IL-8) and G-CSF [181920].
The main objective of the present study is to compare the potential differences in the expressions of different CAMs and chemokines on CD34+ cells obtained from the BM before and after CD34+ cell mobilization. Possible differences in the behavior of CAMs between individuals with successful and those with failed mobilization were investigated. We also aimed to compare the behavior of CAMs and chemokines between donors and patients with lymphoma and multiple myeloma who have undergone CD34+ cell mobilization.
A non-randomized prospective protocol involving 48 individuals comprising 20 patients with lymphoma, 16 with multiple myeloma with indication for autologous transplantation, and 12 healthy PBSC donors were included in this study. The study protocol was approved by the Ethics Committees of Universidade Federal de São Paulo (UNIFESP) and Santa Marcelina Hospital. Written informed consent was obtained from all patients, and the study was performed in accordance with the 1975 Helsinki Declaration. All patients were in complete or partial remission before mobilization. The clinical characteristics of these patients are summarized in Table 1. Among the patients with lymphomas, 13, 1, 2, and 4 had diffuse large B cell, anaplastic Tcell, advanced follicular non-Hodgkin's lymphoma, and relapsed Hodgkin's lymphoma, respectively. The 2 patients with non-Hodgkin's lymphoma received 6 to 15 cycles of chemotherapy before mobilization, whereas those with Hodgkin's disease received 9 to 12 cycles. Among the sixteen patients with multiple myeloma, thirteen had advanced disease (11, IIIA; 2, IIIB) and a monoclonal M component (12, IgG kappa; 2, IgG lambda; and 2, light chain) at diagnosis. They received 3 to 18 cycles of chemotherapy before mobilization. The mobilization procedures comprised chemotherapy and subsequent G-CSF administration at a dose of 10 mg/kg daily for the patients and G-CSF alone for the donors. The type of mobilization regimen utilized for each disease and healthy donors are summarized in Table 2.
The target optimal CD34+ cell yield at our institution is at least 5×106/kg recipient body weight, whereas a minimum dose of at least 2×106/kg is recommended to proceed with ASCT. With this, we adopted the criterion to define successful mobilization for those individuals showing both CD34+ cell concentration of at least 15 cells per µL in the PB at the first day of the harvest and a yield of 5×106 per kg at most in up to 4 apheresis.
BM samples before and after HPC mobilization were obtained from each patient and healthy donor. For all patients, PBSCs were mobilized after chemotherapy according to the specific treatment protocol. We administered 10 µ/kg G-CSF (granulokine, Roche, Brazil) subcutaneously twice daily after the white blood cell (WBC) count reached ≤1×109/L, and we maintained this level until the last day of large volume leukapheresis (LVL). The number of CD34+ cells in the PB was counted via flow cytometry to determine the PBSC overshoot for optimal collection. PBSC collection was started on the day the peripheral CD34+ cell count reached 15/µL or higher. The CD34+ cell were mobilized in healthy donors using 10 µg/kg/day G-CSF, administered subcutaneously daily, with the collection of cells on days 5 and 6. LVL was used to process more than 3 volumes of blood in a single session of apheresis [21].
A temporary central venous access using a double-lumen catheter was obtained in 33 individuals in whom peripheral access was not possible. PBSCs were collected using a Cobe-Spectra cell separator (Gambro BCT, Lakewood, CO, USA) under manual control to obtain a final product with hematocrit of 2%. The patient's and donor's blood volumes were calculated using an automatic program incorporated in the separator according to the Nadler-Allen formula that considered weight, height, and gender [22]. Acid-citrate-dextrose (ACD-A, Hilex-Istar, Brazil) was used as an anticoagulant. To prevent hypocalcemia, calcium gluconate was systematically administered before and during collection. After LVL, all patients were evaluated via complete blood count and biochemical tests. Metabolic abnormalities were corrected and blood components were transfused as needed.
The CD34+ cells in the PB, BM, and aliquots of LVL were analyzed via flow cytometry (FACSCalibur BD, San Jose, CA, USA) within 12 hours of collection. Only samples from BMA were subjected to the mononuclear cell (MNC) separation process via phycoll/Hypaque. All samples were incubated with double-marked conjugated monoclonal anti-CD34/CD45 antibodies (CD34PE, Clone 8G12, Pharmingen/CD45 FITC IgG1,anti-HLe-1, Pharmingen) for 20 minutes at 20℃ after lysing solutions were added for 10 min. The samples were washed with buffer saline and fixed with 1% formaldehyde. Flow cytometric analyses were performed according to the ISHAGE protocol, and the data were analyzed with Cell Quest software (Becton Dickinson, Franklin Lakes, NJ, USA) [22].
BM samples obtained from the iliac crests using heparin as the anticoagulant, before and after HPC mobilization, were utilized in this study. After MNC separation, the samples were assessed via flow cytometry within 24 hours after collection. Fluorochromes and monoclonal antibodies, namely, CD45 PerCP-cy5.5 (Clone 2D1 Pharmingen), CD34 PE (Clone 581 Immunotech), or FITC (Clone 581 Immunotech), against such the adhesion molecules studied were added to each BM sample. The expression of VCAM-1 (CD106-PE IgG1 kappa, Clone 51-10C9 Pharmingen), FLT-3 (CD135-PE, IgG1 kappa, Clone 4G8 Pharmingen), CXCR4 (CD184-PE, IgG2a kappa, Clone 12G5 Pharmingen), CD44 (CD44-PE IgG1 kappa, Clone 515 Pharmingen), L-selectin (CD62L FITC IgG1, Clone DREG56 Immunotech), VLA-4 (CD49d FITC IgG1, Clone HP2.1, Immunotech), and LFA-1(CD11a FITC IgG1, Clone 25.3.1, Immunotech) was analyzed following the ISHAGE recommendation of using 3-color fluorescence with sequential gating (Fig. 1). Flow cytometric analysis was carried out on a standard FACScan (FACSCalibur BD, San Jose, CA, USA) instrument. Forward scatter and side scatter (SSC) signals were recorded in linear mode and the fluorescence signals in logarithmic mode. The threshold for positive cells was set with an appropriated isotype control, and a minimum of 300,000 events were acquired for analysis of adhesion molecules and chemokine staining. A minimum of 100 events within the acquisition gate were stored. The amount of adhesion molecules expressed on the CD34+ population was calculated using correction of nonspecific fluorescence from the isotype control. The level of expression was estimated as the mean fluorescence intensity (MFI) in the positive cells. To evaluate the MFI, a histogram plot was used after CD34+ cells have been gated on CD34 fluorescence/SSC (Fig. 2). All data were analyzed using Cell Quest software (Becton Dickinson, Franklin Lakes, NJ, USA).
Nonparametric student's t-test was used to compare the 2 study populations. Analysis of variance between multiple groups was performed using Tukey's multiple comparison test. Results with a P-value ≤0.05 were considered significant. Logistic regression method was applied to investigate the association between the expression of adhesion molecules and the yield of CD34+ cells. All statistical analyses were performed using the SPSS software (SDSS.1.4, 2001, SPSS Inc., Chicago, IL, USA).
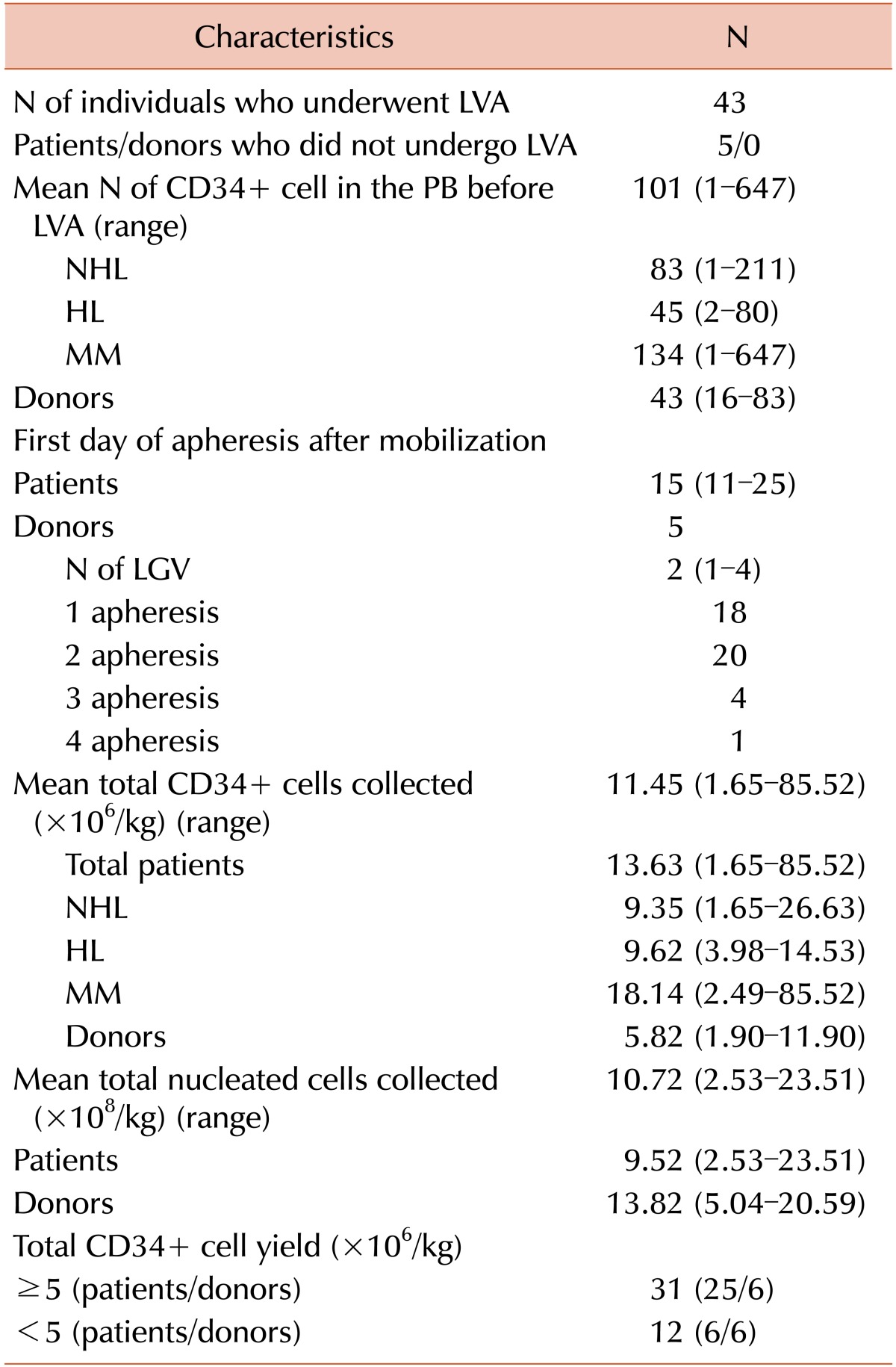
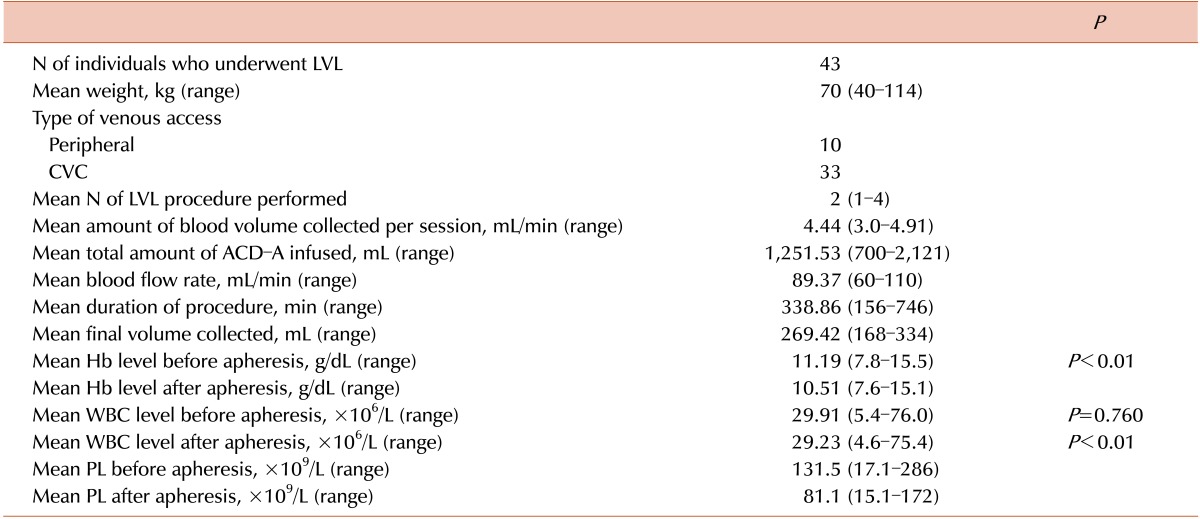
Twelve PBSC donors and 36 patients were enrolled in this study. All patients and healthy donors underwent mobilization therapy as described before (Table 2). The number of CD34+ cells in the PB was determined prior to LVL only in patients. The mean number of CD34+ cells in the PB before LVL was 101.34/µL (range, 1–647.2). The mean time between mobilization chemotherapy and the first day of LVL was 12 days (range, 5–21). A total of 43 individuals comprising 31 patients and 12 donors underwent LVL. Five patients failed to reach the adequate number of CD34+ cells in PB; thus, they did not undergo LVL. The mean yield of CD34+ cells in the AP was 11.45×106/kg (range, 1.65–85.52), and it was higher in the patients than in donors (13.63±2.88 and 5.82±0.75, respectively; P=0.019). MM patients showed the highest number of CD34+ cells in the collected PBSC (mean, 18.14±5.53). Among all study participants who underwent LVL, 31 (25 patients and 6 donors) reached a CD34+ cell yield of ≥5×106/kg, and 12 (6 patients and 6 donors) reached ≤5×106/kg (Table 2). The characteristics of patients who underwent LVL are described in Table 3.
We determined the expressions of CD106, CD135, CD184, CD44, CD62, CD49d, and Cd11a on the CD34+ cells in steady-state BM for both donors and patients. Ours results provide evidence that the number of CD44 and CD49d cells in steady-state BM differs significantly between donors and patients. The mean expression in MFI of CD44 in donors and patients was 834.34±47.01 and 874.31±46.56 (P=0.010), respectively. Meanwhile, the mean expression of CD49d in donors and patients was 495.32±29.57 and 520.84±22.65 (P=0.002), respectively. For all the groups studied, when we analyzed the correlation between CAMs and chemokine expression with good and poor yield of CD34+ cells, we found a statistically significant difference in CD106 (P=0.007), CD44 (P=0.027), and CD49d (P=0.014) (Fig. 3). Those with poor CD34+ yield presented a higher expression of these 3 molecules than those with good CD34+ cell yield.
We determined the expression of CD106, CD135, CD184, CD44, CD62, CD49d, and Cd11a on the CD34+ cells from the BM after HPC mobilization for both donors and patients. We found a reduced expression of the following molecules after HPC mobilization that was independent of the CD34+ yield: CD106 (P=0.001), CD135 (P=0.001), CD44 (P=0.011), CD49d (P=0.001), and CD11a (P=0.001) (Fig. 4).
Meanwhile, CD184 expression was not significantly different before and after mobilization, indicating that its behavior was different. However, for those subjects with better CD34+ yield, CD184 expression varied; it was higher in the pre-mobilization (P=0.002) (Table 4) than post-mobilization, whereas it was not significantly different before and after mobilization among those with worse CD34+ cell yield (P=0.156). CD184 expression was higher in steady-state BM cells among individuals with successful BM aspiration than those with unsuccessful procedure (529.84±54.68 and 496.31±97.51, respectively, P=0.05).
Changes in the PB concentration of CD34+ cells during HPC mobilization are the result of dynamic factors including the rate of egress of CD34+ cells from the marrow, the circulatory volume of CD34+ cell distributed, and the rate of CD34+ cells returning to the marrow. CAMs and chemokines are expressed at different levels in the BM before and after HPC mobilization. We compared the behavior of CAMs and chemokines before and after HPC mobilization independent of the CD34+ yield. Expression of the following molecules was significantly reduced in the BM after mobilization: CD106 (P=0.001), CD49d (P=0.001), CD11a (P=0.001), CD44 (P=0.011), and CD135 (P=0.001). CD106 is constitutively expressed in BM stromal/endothelial cells and certain classes of hematopoietic cells (e.g., B cells, follicular, dendritic cells, and macrophages). CD 106 and fibronectin are essential ligands to CD49d [23]. We found an extremely lower expression of CD106 in the CD34+ cell population before and after mobilization compared with other molecules, and these findings are similar to that previously reported in studies in which the expression of CD106 was significantly higher in the marrow stromal than HPCs [2324]. Our study showed a reduced CD49d expression in the BM after mobilization. CD49d has been implicated in marrow homing and retention of hematopoietic stem cells (HSC) owing to the binding of endothelial and stromal cells on the BM surface [232425]. Furthermore, studies examining GCSF-mediated HSC mobilization from the marrow, a process that is accompanied by significant blood neutrophilia, have shown that its effects on the marrow are partly mediated by the downregulation of CD106, presumably interrupting CD49d adhesion. Many researchers found a reduced activation state and decreased expression of CD49d on circulating CD34+ cells compared with CD34+ cells residing in the BM [242526]. Although our study has not analyzed the expression of CAMs in the PB after HPC mobilization, the reduced CD49d expression on CD34+ cells in the BM after mobilization suggests that CD49d is critically involved in the regulation of CD34+ cell trafficking, and it is associated with a facilitated egress and circulation of HPCs. These findings are in accordance with the previous reports that G-CSF-stimulated HPC mobilization results in increased levels of circulating endothelial adhesion molecules. [2526]. It is possible that an impaired BM microenvironment does not release proteolytic enzymes in parallel amount from neutrophil precursors. This damaged microenvironment may retain a higher amount of HPCs, particularly in heavily treated patients. This hypothesis is supported by the lower CD49d expression after BM mobilization in healthy donors than in previously treated patients in our study (495.32±29.57 and 520.84±22.65, respectively; P=0.002).
The β2 integrins CD11a and macrophage antigen-1 have been reported to play a role in the attachment of CD34 cells to stromal cells through one of their ligands, that is, ICAM-1 CD54 and heparan sulfate [27]. CD11a expression was lower in the BM after mobilization than before mobilization. We did not find any correlation between good yield of CD34+ and CD11a expression. A possible role of CD11a and CD18 during mobilization was described by Gunji et al. [28], who reported a significantly decreasing number of colony-forming units generated compared to that in controls on using anti-CD-11a and anti-CD18 antibodies in a co-culture system of CD34+CD33− cells with a stromal layer.
CD44 mediates adhesion of HA to HSC, and its importance as an adhesion molecule for HSC has been demonstrated [2930]. Herein, the mean expression of CD44 in donors and patients before mobilization was 834.34±47.01 and 874.31±46.56, respectively (P=0.010). In the present study, CD44 expression was significantly correlated with CD34+ yield (P=0.014). CD44 expression was higher among those with poor CD34+ yield than those with good yield. Our results show that the correlation of CD44 expression in the BM before mobilization with CD34+ yield can be related to the outcome of cellular migration, suggesting that high levels of CD44 lead to a condition where the cells remain adhered to the microenvironment, thus leading to a decrease in HPC mobilization. Similar to our results, Lee et al. [31] found a downregulation of CD44 expression after G-CSF administration.
FLT3 is produced by stroma cells and is a strong stimulator of the lympho-hematopoietic progenitors [3233]. Because of the relevant involvement of FLT3 in the hematopoiesis and to further assess the possible impact of the administration of this cytokine in the context of stem cell transplantation, we studied the influence of FLT3 to mobilization before and after chemotherapy followed by G-CSF administration. In both patients and donors, the MFI of CD34+ cells was higher before mobilization than after (P=0.001), but we did not find any correlation between FLT3 and the yield of CD34+ cells collected via LVL. Our results can be explained by the downregulation of FLT3 on mobilized CD34+ cells, indicating the role of FLT3 in the migration of HPCs. Fukuda et al. [32] showed compelling evidence that the FL/FLT3 axis also regulates to the migration of normal and transformed hematopoietic cells and that this effect is mediated through the CXCL12/CXCR4 axis. FLT3L is a stem-cell specific growth factor that expands and may also mobilize stem cells in mice after its administration for 10 days either as a single agent or in combination with other molecules such as IL-8 and G-CSF [33]. Recently, a clinical trial of FLT3L showed that its administration for 5 or 10 consecutive days effectively mobilizes CD34+ HSCs in healthy donors [34].
The CXCR4 expression on CD34+ cells analyzed in BMA before and after mobilization was statistically significantly different among individuals with a good yield of CD34+ cells (P=0.036). Interestingly, this was not observed among those with poor yield (P=0.156). We also found that CD184 in steady-state BMA was higher among individuals with successful LVL than those with unsuccessful procedure (529.84±54.68 and 496.31±97.51, respectively; P=0.05). The roles of SDF-1 and CXCR4 in BM progenitor cell retention and release are well established. Selective antagonism of CXCR4 with the pharmacological agent AMD3100 (plerixafor) rapidly and potently mobilizes BM progenitor cells in both animals and humans [35]. Both the release of progenitor cells from the BM to the peripheral blood and the recruitment and retention of progenitor cells in ischemic tissue are regulated by interactions between SDF-1 and CXCR4. The rationality for our findings is based on the functionality of the CXCR4 receptor on HPCs that is modulated by several factors as follows: 1) the level of receptor expression on the cell surface [35363738]; 2) the sulfation status of its N-terminus [35363738]; 3) the availability of SDF-1 [363738]; 4) the cleavage of the CXCR4 N-terminus on the cells and SDF-1 in extracellular space by serine proteases and metalloproteinase-9 [36]. Administration of mobilizing agents of CD34+ cell leads to the inactivation of the SDF-1/CXCR4 axis and downregulation of expression of this molecule in the BM, facilitating the egress of HPCs into the circulation [363738].
In conclusion, we attempted to determine particular aspects of CAMs and chemokines involved in the mobilization of CD34+ cells. However, the mechanism of mobilization is highly complex and involves adhesion molecules. Moreover, the interaction between different matrix metalloproteases and the mechanism by which they are activated through proteolytic enzymes are not fully understood. We believe that CXCR4, VLA-4, CD44, and VCAM-1 are the most important molecules involved in HPC mobilization because they showed correlation with the CD34+ yield via LVL. Determining predictive markers for mobilization exclusively through laboratory methods is difficult. Among the molecules studied, CD44 and VLA-4 had the highest potentials (Fig. 5). In particular, CD44 expression was significantly high in heavily treated patients. Further studies are needed to validate our results.
Notes
References
1. Arslan O, Moog R. Mobilization of peripheral blood stem cells. Transfus Apher Sci. 2007; 37:179–185. PMID: 17980665.

2. Ikeda K, Kozuka T, Harada M. Factors for PBPC collection efficiency and collection predictors. Transfus Apher Sci. 2004; 31:245–259. PMID: 15556472.

3. Haverkos BM, McBride A, O'Donnell L, et al. An effective mobilization strategy for lymphoma patients after failed upfront mobilization with plerixafor. Bone Marrow Transplant. 2014; 49:1052–1055. PMID: 24797182.

4. Angelopoulou MK, Tsirkinidis P, Boutsikas G, Vassilakopoulos TP, Tsirigotis P. New insights in the mobilization of hematopoietic stem cells in lymphoma and multiple myeloma patients. Biomed Res Int. 2014; 2014:835138. PMID: 25197663.

5. Yuan S, Wang S. How do we mobilize and collect autologous peripheral blood stem cells? Transfusion. 2017; 57:13–23. PMID: 27731496.

6. Olivieri A, Marchetti M, Lemoli R, et al. Proposed definition of ‘poor mobilizer’ in lymphoma and multiple myeloma: an analytic hierarchy process by ad hoc working group Gruppo ItalianoTrapianto di Midollo Osseo. Bone Marrow Transplant. 2012; 47:342–351. PMID: 21625224.

7. Fruehauf S, Seggewiss R. It's moving day: factors affecting peripheral blood stem cell mobilization and strategies for improvement. Br J Haematol. 2003; 122:360–375. PMID: 12877663.
8. Kröger N, Zeller W, Hassan HT, Dierlamm J, Zander AR. Difference between expression of adhesion molecules on CD34+ cells from bone marrow and G-CSF-stimulated peripheral blood. Stem Cells. 1998; 16:49–53. PMID: 9474747.
9. Chan JY, Watt SM. Adhesion receptors on haematopoietic progenitor cells. Br J Haematol. 2001; 112:541–557. PMID: 11260052.

10. Ford CD, Greenwood J, Anderson J, Snow G, Petersen FB. CD34+ cell adhesion molecule profiles differ between patients mobilized with granulocyte-colony-stimulating factor alone and chemotherapy followed by granulocyte-colony-stimulating factor. Transfusion. 2006; 46:193–198. PMID: 16441594.

11. Oostendorp RA, Reisbach G, Spitzer E, et al. VLA-4 and VCAM-1 are the principal adhesion molecules involved in the interaction between blast colony-forming cells and bone marrow stromal cells. Br J Haematol. 1995; 91:275–284. PMID: 8547062.

12. Lichterfeld M, Martin S, Burkly L, Haas R, Kronenwett R. Mobilization of CD34+ haematopoietic stem cells is associated with a functional inactivation of the integrin very late antigen 4. Br J Haematol. 2000; 110:71–81. PMID: 10930981.
13. Lévesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003; 111:187–196. PMID: 12531874.

14. Devine SM, Flomenberg N, Vesole DH, et al. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin's lymphoma. J Clin Oncol. 2004; 22:1095–1102. PMID: 15020611.

15. Girbl T, Lunzer V, Greil R, Namberger K, Hartmann TN. The CXCR4 and adhesion molecule expression of CD34+ hematopoietic cells mobilized by “on-demand” addition of plerixafor to granulocyte-colony-stimulating factor. Transfusion. 2014; 54:2325–2335. PMID: 24673458.
16. Avigdor A, Goichberg P, Shivtiel S, et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004; 103:2981–2989. PMID: 15070674.

17. Szmigielska-Kaplon A, Szemraj J, Hamara K, et al. Polymorphism of CD44 influences the efficacy of CD34(+) cells mobilization in patients with hematological malignancies. Biol Blood Marrow Transplant. 2014; 20:986–991. PMID: 24680978.

18. Bojko P, Pawloski D, Stellberg W, Schröder JK, Seeber S. Flt3 ligand and thrombopoietin serum levels during peripheral blood stem cell mobilization with chemotherapy and recombinant human glycosylated granulocyte colony-stimulating factor (rhu-G-CSF, lenograstim) and after high-dose chemotherapy. Ann Hematol. 2002; 81:522–528. PMID: 12373354.
19. Papayannopoulou T, Nakamoto B, Andrews RG, Lyman SD, Lee MY. In vivo effects of Flt3/Flk2 ligand on mobilization of hematopoietic progenitors in primates and potent synergistic enhancement with granulocyte colony-stimulating factor. Blood. 1997; 90:620–629. PMID: 9226162.

20. He S, Chu J, Vasu S, et al. FLT3L and plerixafor combination increases hematopoietic stem cell mobilization and leads to improved transplantation outcome. Biol Blood Marrow Transplant. 2014; 20:309–313. PMID: 24365795.

21. Zubair AC, Rymer R, Young J, et al. Multiple myeloma patients receiving large volume leukapheresis efficiently yield enough CD34+ cells to allow double transplants. J Clin Apher. 2009; 24:6–11. PMID: 19156756.

22. Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996; 5:213–226. PMID: 8817388.
23. Petty JM, Lenox CC, Weiss DJ, Poynter ME, Suratt BT. Crosstalk between CXCR4/stromal derived factor-1 and VLA-4/VCAM-1 pathways regulates neutrophil retention in the bone marrow. J Immunol. 2009; 182:604–612. PMID: 19109194.

24. Südhoff T, Söhngen D. Circulating endothelial adhesion molecules (sE-selectin, sVCAM-1 and sICAM-1) during rHuG-CSF-stimulated stem cell mobilization. J Hematother Stem Cell Res. 2002; 11:147–151. PMID: 11847011.

25. Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: dominant role of alpha(4)beta(1) over beta(2)-integrins and selectins. Blood. 2001; 98:2403–2411. PMID: 11588037.
26. Lévesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001; 98:1289. PMID: 11520773.
27. Velders GA, Pruijt JF, Verzaal P, et al. Enhancement of G-CSF-induced stem cell mobilization by antibodies against the beta 2 integrins LFA-1 and Mac-1. Blood. 2002; 100:327–333. PMID: 12070044.
28. Gunji Y, Nakamura M, Hagiwara T, et al. Expression and function of adhesion molecules on human hematopoietic stem cells: CD34+ LFA-1- cells are more primitive than CD34+ LFA-1+ cells. Blood. 1992; 80:429–436. PMID: 1378320.

29. Zöller M. CD44, hyaluronan, the hematopoietic stem cell, and leukemia-initiating cells. Front Immunol. 2015; 6:235. PMID: 26074915.

30. Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002; 30:973–981. PMID: 12225788.
31. Lee S, Im SA, Yoo ES, et al. Mobilization kinetics of CD34(+) cells in association with modulation of CD44 and CD31 expression during continuous intravenous administration of G-CSF in normal donors. Stem Cells. 2000; 18:281–286. PMID: 10924094.
32. Fukuda S, Broxmeyer HE, Pelus LM. Flt3 ligand and the Flt3 receptor regulate hematopoietic cell migration by modulating the SDF-1alpha(CXCL12)/CXCR4 axis. Blood. 2005; 105:3117–3126. PMID: 15618475.
33. He S, Chu J, Vasu S, et al. FLT3L and plerixafor combination increases hematopoietic stem cell mobilization and leads to improved transplantation outcome. Biol Blood Marrow Transplant. 2014; 20:309–313. PMID: 24365795.

34. Anandasabapathy N, Hurley A, Breton G, et al. A phase 1 trial of the hematopoietic growth factor CDX301 (rhuFlt3L) in healthy volunteers. Biol Blood Marrow Transplant. 2013; 19(Suppl):S112. (BMT Tandem Meetings Abstracts).

35. Cheng M, Qin G. Progenitor cell mobilization and recruitment: SDF-1, CXCR4, α4-integrin, and c-kit. Prog Mol Biol Transl Sci. 2012; 111:243–264. PMID: 22917234.

36. Carion A, Benboubker L, Hérault O, et al. Stromal-derived factor 1 and matrix metalloproteinase 9 levels in bone marrow and peripheral blood of patients mobilized by granulocyte colony-stimulating factor and chemotherapy. Relationship with mobilizing capacity of haematopoietic progenitor cells. Br J Haematol. 2003; 122:918–926. PMID: 12956762.

37. Dar A, Kollet O, Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol. 2006; 34:967–975. PMID: 16863903.

38. Donahue RE, Jin P, Bonifacino AC, et al. Plerixafor (AMD3100) and granulocyte colony-stimulating factor (G-CSF) mobilize different CD34+ cell populations based on global gene and microRNA expression signatures. Blood. 2009; 114:2530–2541. PMID: 19602709.

Fig. 1
Representative flow cytometric scattergrams from bone marrow aspirate specimens after HPC mobilization showing a 3-color cytofluorometric analysis of the expression of adhesion molecule antigens on CD34+ cells population. (A) CD34+ cells are painted in the lympho-mononuclear region. (B) CD34PE+ cells are included in R1 in the FSC/SSC dot plot. (C) CD34PE+ cells population in R2. (D) CD34FITC+ cells are included in R1 in the FSC/SSC dot plot. (E) CD34FITC+ cells population in R3. (F) CD34PE+ cells are included in R6 in the CD45PerCP/SSC dot plot. (G) CD34FITC+ cells are included in R6 in the CD45PerCP/SSC dot plot.
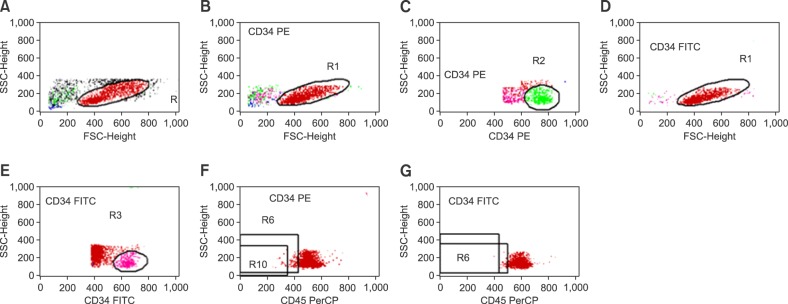
Fig. 2
Representative flow cytometric histograms from bone marrow aspirate specimens after HPC mobilization showing a 3-color cytofluorometric analysis of the expression of adhesion molecule antigen on CD34+ cell population. The results were estimated as the mean fluorescence intensity (MFI). The histograms (A) and (B) are IgG1 FITC and PE controls. (C) CD106, (D) CD135, (E) CD184, (F) CD62L, (G) CD49d, (H) CD11a, and (I) CD44 antigen on CD34+ cell population. The gating protocols have been described before.
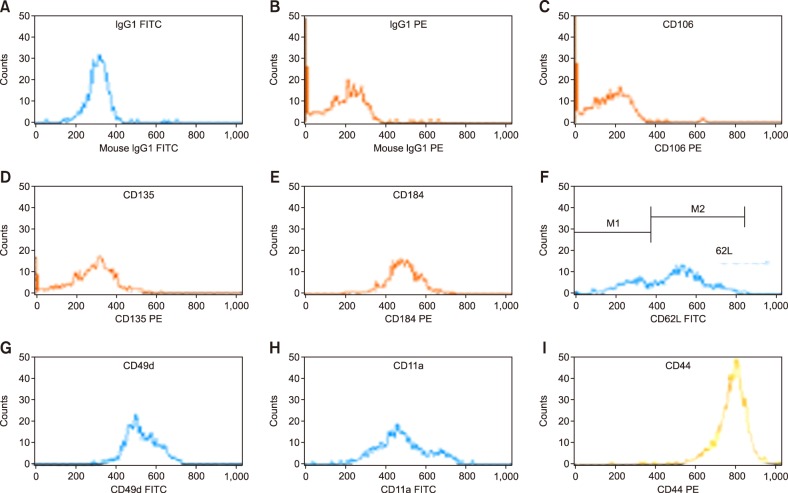
Fig. 3
The mean expression of CD106, CD-44, and CD49d on the CD34+ cells assessed in BMA before mobilization and its association with the yield of CD34+ cells collected via LVL in both donors and patients.
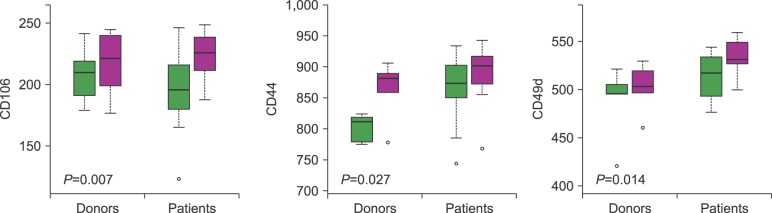
Fig. 4
Distribution of CD106, CD135, CD11a, CD44, CD49d, and CD184 assessed in mononuclear cells of the BMA, before and after mobilization according to the yield of CD34+ cells obtained via LGV.
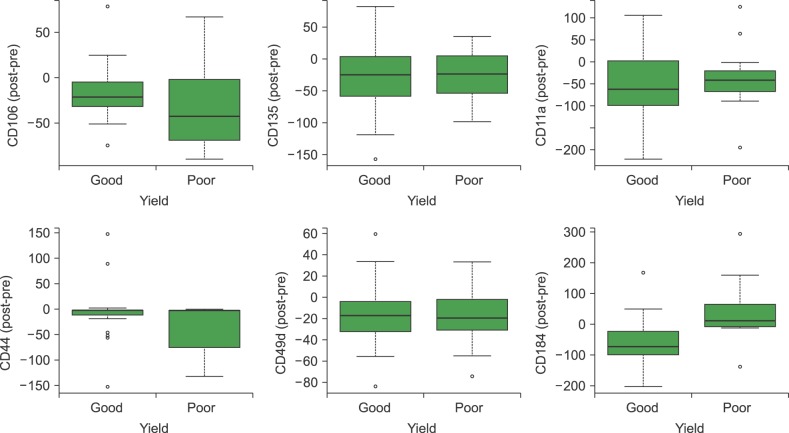
Fig. 5
Results obtained through logistic regression analysis. The chance of poor mobilization increases by approximately 6% with each increase of one unit of CD49d and by approximately 4% with each increase of one unit of CD44 in the pre-mobilization phase.
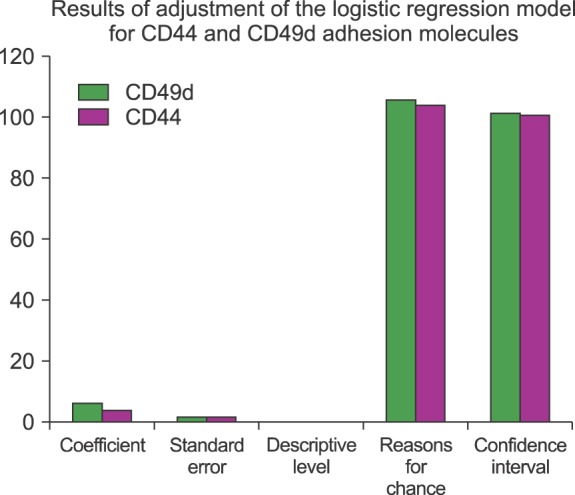
Table 1
Characteristics of the patients and donors.
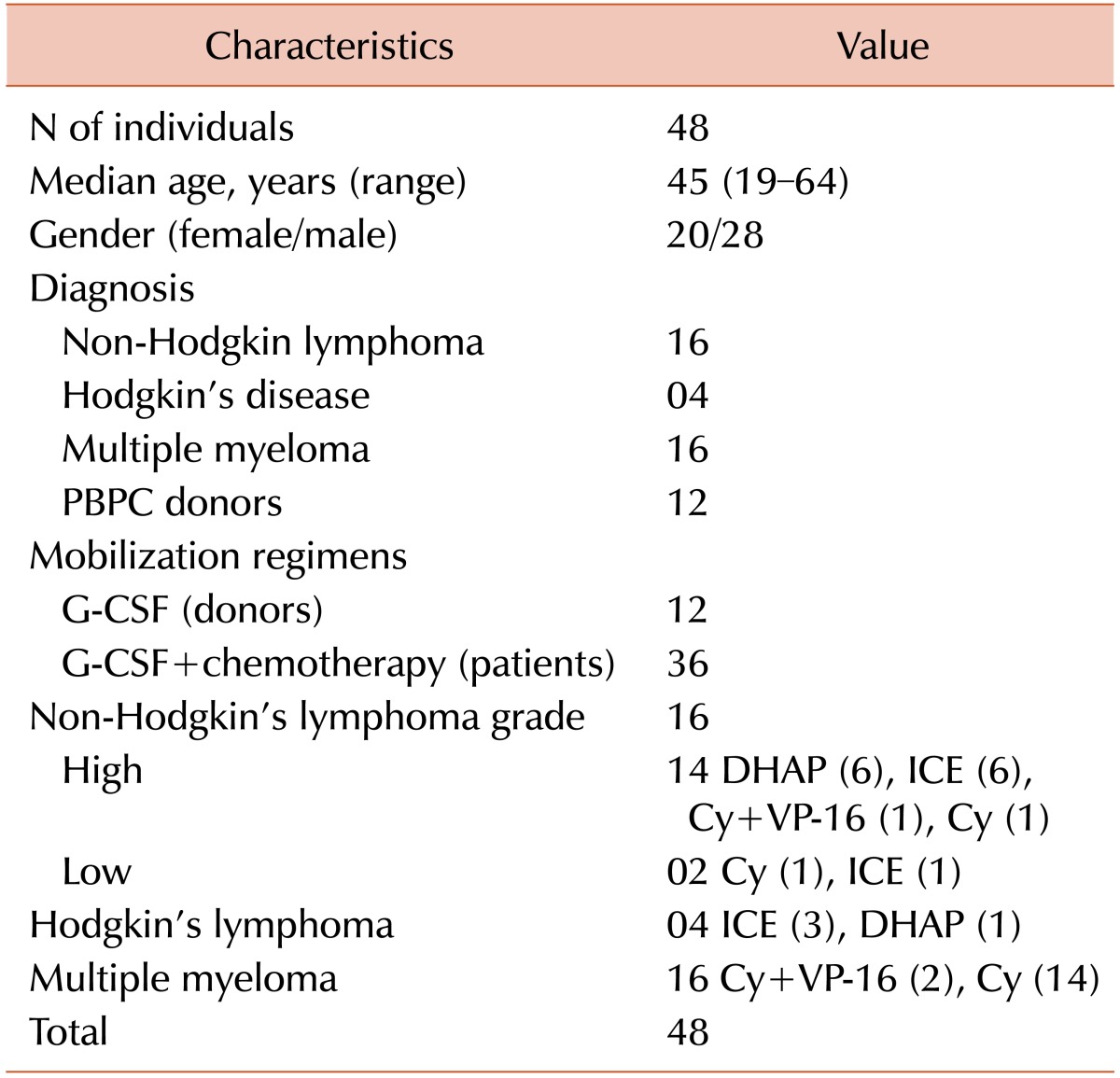
PBPC, peripheral blood progenitors cells.
G-CSF, granulocyte-colony stimulating factor (10 µg/kg/day); Cy-Cyclophosphamide (5 g/m2); Cy+VP-16 Cyclophosphamide (5 g/m2)+VP-16 (400 mg/m2).
ICE, Ifosfamide 5,000 mg/m2 carboplatin AUC=5 (max. 800 mg); e, 100 mg/m2.
DHAP, Cisplatin 100 mg/m2; cytarabine, 2,000 mg/m2; Dexamethasone 40 mg.
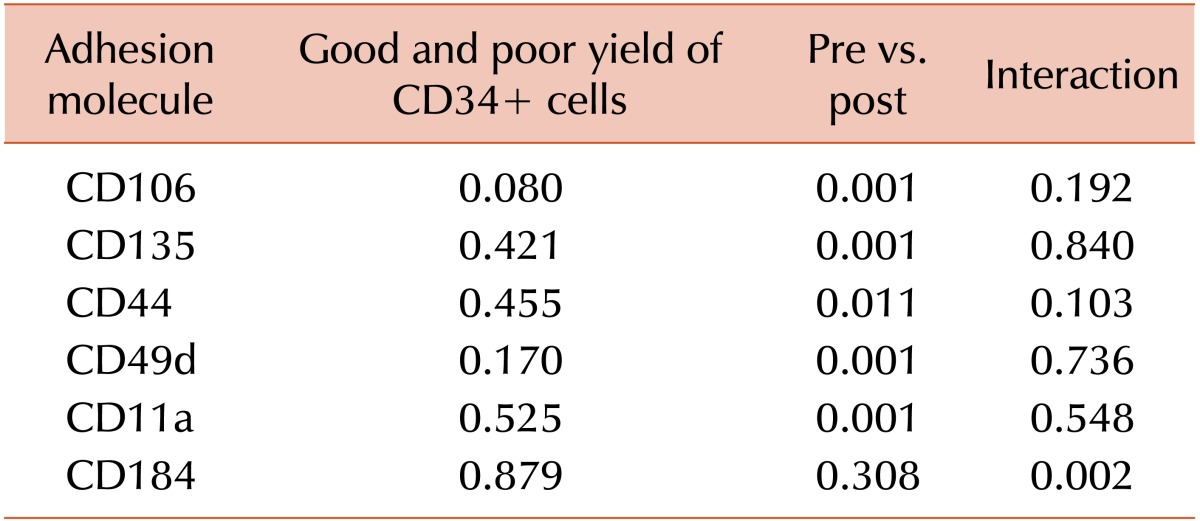
Table 4
Comparative analysis of CAM expression assessed in BMA before and after HPC mobilization, considering good and poor yield of CD34+ cells in LVL. The expression of CD106, CD135, CD44, CD49d, and CD11a varied before and after mobilization, and this was independent of good CD34+ cell yield. Meanwhile, CD184 expression before and after mobilization was only different among those with a good yield of CD34+ cells (P=0.002).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download