Abstract
Background
The numerous side effects and chemo-resistance of conventional chemical drugs in the treatment of malignancies have led to consideration of the anti-cancer properties of natural products. In the present study, we aimed to explore the apoptotic effect of two natural components, resveratrol and prednisolone, on the T acute lymphoblastic leukemia (ALL) cell line, CCRF-CEM. Our findings suggested the incorporation of these natural agents into drug regimens to treat patients with ALL.
Methods
In this study, we investigated the effect of different doses of resveratrol (15, 50 and 100 µM) and prednisolone (700 µM) on BAX (apoptosis promoter) and BCL2 (apoptosis inhibitor) expressions following 24 and 48 hours of treatment on CCRF-CEM cells, using real-time PCR, and on apoptosis induction using flow cytometry.
Results
The results showed a time- and dose-dependent increase in BAX expression and a decrease in BCL2 expression. Apoptosis was induced in CCRF-CEM cells treated with resveratrol and prednisolone for 24 and 48 hours. Combined resveratrol and prednisolone treatment showed synergistic effects on the overexpression of BAX and the downregulation of BCL2. The drug combination had a greater influence on apoptosis induction compared with either drug administered alone after 48 hours of treatment.
Acute lymphoblastic leukemia (ALL) has a high relapse rate, and chemo-resistance is a major characteristic of the disease [12]. One of the main factors affecting a clinical result in ALL is cellular drug sensitivity. Currently, glucocorticoids (GCs) are the most effective drugs to treat pediatric ALL. In the treatment of ALL, early response to prednisolone (a GC) is one of the most helpful prognostic factors [3]. Moreover, GC resistance in patients with ALL leads to treatment failure and consequent relapse [45]. Therefore, by targeting dominant markers of cancers, such as tumor cell resistance, chemo-resistance, and late relapses, survival of patients with cancer can be improved [6]. Deficiency or resistance to apoptosis are hallmarks of cancer and are caused by overexpression of anti-apoptotic proteins [7]. Overexpression of anti-apoptotic proteins such as BCL-2, BCLXL, and MCL-1 has been demonstrated in ALL cell lines. Moreover, the upregulation of BCL2 gene expression levels can help ALL cells to survive, even in the absence of optimal conditions for cell culture; for example, a lack of stromal derived growth factors [8910]. Therefore, an attractive approach to treat ALL is the inhibition of the anti-apoptotic/pro-survival members of the BCL-2 family of proteins. Resveratrol (3, 4, 5-trihydoxystilbene) is a member of the phytoalexins, which are found in many food products, such as grapes, berries, and peanuts. Several plants have been introduced as a source of resveratrol, and certain factors, such as UV irradiation, stress, and injury, can trigger the production of this compound [11]. Previous studies revealed certain interesting medical properties of resveratrol, including cardio-protective, anti-inflammatory, anti-oxidant, and growth-inhibitory activities [1213141516]. Resveratrol can overcome chemo-resistance in tumor cells. This property of resveratrol has been linked to controlling apoptotic pathways, modulating drug transporters, and downregulation of tumor cell proliferation proteins [17]. BCL2 family members, such as BCL-2, BCL-XL, MCL, BCL-W, AL/BFL-1, and BCL-B/BCL-2L10 are pro-survival factors that prevent tumor cells from undergoing apoptosis, while BAX, BAK, and BOK/MTD encode pro-apoptotic proteins that are responsible for inducing cell death [18]. Impairment of the pro-apoptotic and anti-apoptotic balance leads to disruption of apoptosis pathways in the affected cells. It has been widely proposed that frequent overexpression of BCL2 mRNA, combined with downregulation of BAX mRNA, in malignant conditions is one of the underlying mechanisms in pathogenesis of ALL [19]. In this study, we analyzed the effect of different doses of resveratrol (15, 50, and 100 µM), and prednisolone (700 µM), separately, and as a combined treatment, on the expression levels of BAX (pro-apoptotic) and BCL2 (anti-apoptotic) in the ALL cell line, CCRF-CEM. We also evaluated the effect of resveratrol and prednisolone on apoptosis in this cell line.
T-ALL CCRF-CEM cells were purchased from Pasteur Institute of Iran (Tehran, Iran), and cultured at 4×105 cells per dish in Roswell Park Memorial Institute (RPMI) 1640 medium (GIBCO/Invitrogen, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (GIBCO), 50 µg/mL streptomycin, and 50 µg/mL penicillin, and maintained in a humidified incubator in 5% CO2 at 37℃.
Resveratrol and prednisolone (98% purity, Sigma-Aldrich, Germany) were dissolved in ethanol. CCRF-CEM cells were treated separately and in combination with resveratrol (concentrations of 15, 50 and 100 µM) and prednisolone (700 µM). After 12, 24, and 48 hours, the cells were collected for RNA extraction.
Isolation of total RNA was performed using an RNX Plus kit (Cinnagen, Tehran, Iran) according to the manufacturer's protocol. Complementary DNA (cDNA) was synthesized from total RNA using a cDNA synthesis kit (Bioneer, Daejeon, Korea) following the manufacturer's instruction.
The expression levels of BAX and BCL2 were determined using a SYBR Green qRT-PCR kit (Bioneer, Daejeon, Korea) according to the manufacturer's protocol. PCR reactions were performed using specific primers for BAX, BCL2, and the β-actin gene (ACTB) (Takapouzist, Tehran, Iran). The β-actin gene was used as the housekeeping gene to normalize the gene expression values. For graphing purposes, the relative gene expression in untreated cells, as a control, was set to 1. The following primers were used: BAX forward primer: 5′-GCCCTTTTGCTTCAGGGTTT-3′; BAX Reverse primer: 5′-TCCAATGTCCAGCCCATGAT-3′; BCL2 Forward primer: 5′-CGGAGGCTGGGATGCCTTTG-3′; BCL2 Reverse primer: 5′-TTTGGGGCAGGCATGTTGAC-3′; ACTB Forward primer: 5′-GAGACCTTCAACACCCCAGCC-3′; ACTB Revers primer: 5′-AGACGCAGGATGGCATGGG-3′. The reactions were carried out in triplicate using an Eco Real-Time PCR system (Illumina, San Diego, CA, USA).
Cell death was assessed by Annexin V and Propidium Iodide (PI) staining (Exbio, Vestec, Czech Republic) according to the manufacturer's protocol. The cells were analyzed using a fluorescence activated cell sorting (FACS) caliber Flow cytometer (BD, San Jose, CA, USA) to determine the percentage of apoptosis.
The statistical calculations were performed using GraphPad Prism software (GraphPad Prism software Inc., La Jolla, CA, USA). The Kruskal-Wallis test and Dunn's test were used as multiple comparison tests to compare the statistical differences between more than two groups. P<0.05 was considered statistically significant.
The expression levels of BAX and BCL2 were assessed in cultured T-ALL CCRF-CEM cell lines at 15, 50, and 100 µM concentrations of resveratrol and/or 700 µM of prednisolone versus untreated cells, using qRT PCR. No significant changes in the expression levels of BAX and BCL2 were observed at 12 hours. After 24 hours of treatment, the mRNA levels of BAX increased significantly under treatment with resveratrol at 100 µM, prednisolone at 700 µM, and a combination of resveratrol (50 µM) and prednisolone (700 µM). Only the combined treatment significantly decreased the mRNA levels of BCL2. The expression of BCL2 remained unchanged in the other conditions. Except for 15 µM resveratrol, we observed a significant increase in the mRNA level of BAX after 48 hours of treatment under all tested conditions. After 48 hours, a significant decrease in the expression of BCL2 was observed only using the combined treatment of resveratrol (RES) and prednisolone (PRE) at the following doses: 50 µM RES+700 µM PRE (Fig. 1, 2).
Apoptosis evaluation in CCRF-CEM cells treated with combined and separate treatment of resveratrol and prednisolone was performed using flow cytometry after 24 and 48 hours of treatment (Fig. 3, 4, 5). Ethanol-treated cells were used as the vehicle control. According to the obtained data, treatment with different doses of resveratrol and prednisolone significantly induced apoptosis in a time and dose-dependent manner in CCRF-CEM cells (Fig. 3A, B).
ALL is the most common pediatric cancer, which initiates from the bone marrow, followed by progressive invasion into other tissues [20]. Reduction of apoptosis or resistance to apoptotic pathways plays a critical role in pathogenesis of ALL. There are several ways to reduce or resist apoptosis in malignant cells. Consequently, to achieve optimal outcomes, stimulation of apoptosis is the therapeutic target of many drugs [20]. Knowledge of the molecular pathways that contribute to the development of leukemia might guide the development of new treatments to improve patient survival [21]. The overexpression of BCL2 in almost all types and subtypes of leukemia reveals the critical role of this protein in the pathogenesis and development of the disease. Although BCL2 is the most well-known member of the family, data indicate that other BCL2 family proteins, such as BAX, also play key roles [21]. In the present study, we examined the effect of resveratrol and prednisolone on BAX and BCL2 expression levels in ALL CCRF-CEM cells. Based on the qRT-PCR results, we observed that treatment time had a critical role in the effect of resveratrol; however, the effect of treatment time was not observed in cells treated with prednisolone. CCRF-CEM cells are a chemo-resistant cell line and perhaps for this reason, they did not show the expected apoptosis induction over the time course of the experiment. Currently, efforts to improve the survival of patients with cancer are based on approaches targeting chemotherapy resistance (chemo-resistance) and relapse, which are typical characteristics of ALL [22]. Resveratrol has diverse biological effects on cancerous cells, such as anti-proliferative, anti-inflammatory, and chemo protective activities [121314]. Some in vitro studies have demonstrated the potency of resveratrol to provoke apoptosis and inhibit the proliferation of a diversity of human cancer cell lines [23]. Recent research has described the chemo-prevention effect of resveratrol in combination with other chemotherapeutic drugs or cytotoxic factors in drug resistant tumor cells [13]. The anti-carcinogenic effect of resveratrol seems to be linked to its anti-oxidant property. It was revealed that this compound inhibits cyclooxygenase, hydroperoxidase, protein kinase C, BCL2 phosphorylation, Akt, a kinase binding position (focal adhesion kinase), nuclear factor-kappa B (NF-κB), matrix metalloprotease-9, and regulators of the cell cycle. Jazirehi and Bonavida [24] demonstrated the synergistic apoptotic effect of resveratrol and paclitaxel to selectively modify the expression levels of regulatory proteins in the apoptotic signaling pathway in resistant non-Hodgkin lymphoma and multiple myeloma cell lines. Fulda and Debatin [25] reported that resveratrol is an effective sensitizer for chemotherapeutic agents that induce apoptosis, such as doxorubicin, cytarabine (AraC), actinomycin D, paclitaxel (Taxol), and methotrexate, in a variety of human tumor cell lines via induction of cell cycle arrest and downregulation of Survivin expression. Bhardwaj et al. [26] showed that resveratrol could overcome chemo-resistance by downregulation of the NF-κB and signal transducer and activator of transcription 3 (STAT3) pathways. By inhibition of the NF-κB and STAT3 signaling pathways, resveratrol increases the release of BAX and the activation of caspase-3, which led to improvement of the apoptotic and anti-proliferative activities of Velcade and thalidomide in multiple myeloma cells. That study reported that resveratrol caused downregulation of various proliferative and anti-apoptotic gene products, such as Cyclin D1, cIAP-2, XIAP, Survivin, BCL-2, Bcl-XL, BFL-1/A1, and TRAF2. An elevated ratio of BAX/BCL2 was also reported previously in leukemia cell lines CEM-C7H2 and Jurkat, as a result of resveratrol treatment, suggesting that it exerts its pro-apoptotic effects by influencing intrinsic pathways [27]. Resveratrol induces apoptosis in B acute lymphoblastic leukemia, which is resistant to CD95-dependent signal transduction through the mitochondrial/caspase-9 pathway, as well as via the CD95-independent pathway [28]. Another study reported that resveratrol inhibited proliferation of two cell lines (OCMI2 and OCI/AML3) by arresting the cells in S phase [29]. By contrast, Cecchinato et al. [30] demonstrated that resveratrol upregulates the levels of the pro-apoptotic protein p53, inhibits the PI3K/Akt pathway, activates GSK-3β, and inhibits the NOTCH survival signaling pathways in MOLT-4 ALL cells. At a result, resveratrol induced apoptosis in a time- and dose-dependent manner in MOLT-4 cells by inhibiting the NOTCH signaling pathway and interfering with the p53 and PI3K/Akt pathways. Based on these previous studies, we aimed to combine resveratrol as a supplementary drug with prednisolone as a first line treatment. We did not achieve the expected results at 12 and 24 hours after treatment with the combined dose of resveratrol and prednisolone. However, after 48 hours, we observed that the combined treatment significantly increased the induction of apoptosis. The qRT-PCR result was in complete accord with the observed induction of apoptosis using the combined treatment. In the present study, we selected two doses of resveratrol (50 and 100 µM) to combine with prednisolone. Resveratrol at 15 µM was not combined with prednisolone because of its low effectiveness. One of the problems that we encountered was the percentage of cell necrosis that was observed in combined treatment of prednisolone and 100 µM of resveratrol. Hence, we used 50 µM of resveratrol as a selected and final dose to combine with prednisolone for gene expression assessment. Ultimately, our findings confirmed that resveratrol caused overexpression of BAX, decreased the expression of BCL2, and induced apoptosis in dose- and time-dependent manners via regulation of anti-apoptotic and pro-apoptotic proteins of the BCL-2 family. In addition, the flow cytometry results showed that resveratrol could induce apoptosis in a dose- and time-dependent manner in the CCRF-CEM cell line. In addition, treatment of CCRF-CEM cells with the combination of resveratrol and prednisolone confirmed the synergistic effect of these two compounds.
In conclusion, flow cytometry and RT-PCR assays showed that resveratrol could enhance the effects of a glucocorticoid drug (prednisolone) in the treatment of ALL.
ACKNOWLEDGMENTS
This article is based on data from a M.Sc. thesis registered at Tabriz University of Medical Sciences and we appreciate the support from the Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
References
1. Faderl S, O'Brien S, Pui CH, et al. Adult acute lymphoblastic leukemia: concepts and strategies. Cancer. 2010; 116:1165–1176. PMID: 20101737.
2. Tafuri A, Milella M, Iacovelli S, et al. Aberrant proliferative and apoptotic pathways in acute lymphoblastic leukemia (ALL): Molecular therapies to overcome chemo-resistance. In : Stefan Faderl, editor. Novel aspects in acute lymphoblastic leukemia. Rijeka, Croatia: InTech;2011. p. 183–210.
3. Dördelmann M, Reiter A, Borkhardt A, et al. Prednisone response is the strongest predictor of treatment outcome in infant acute lymphoblastic leukemia. Blood. 1999; 94:1209–1217. PMID: 10438708.
4. Klumper E, Pieters R, Veerman AJ, et al. In vitro cellular drug resistance in children with relapsed/refractory acute lymphoblastic leukemia. Blood. 1995; 86:3861–3868. PMID: 7579354.

5. Kaspers GJ, Wijnands JJ, Hartmann R, et al. Immunophenotypic cell lineage and in vitro cellular drug resistance in childhood relapsed acute lymphoblastic leukaemia. Eur J Cancer. 2005; 41:1300–1303. PMID: 15869873.

6. Beesley AH, Firth MJ, Ford J, et al. Glucocorticoid resistance in T-lineage acute lymphoblastic leukaemia is associated with a proliferative metabolism. Br J Cancer. 2009; 100:1926–1936. PMID: 19436302.

8. Hogarth LA, Hall AG. Increased BAX expression is associated with an increased risk of relapse in childhood acute lymphocytic leukemia. Blood. 1999; 93:2671–2678. PMID: 10194447.

9. Campana D, Coustan-Smith E, Manabe A, et al. Prolonged survival of B-lineage acute lymphoblastic leukemia cells is accompanied by overexpression of bcl-2 protein. Blood. 1993; 81:1025–1031. PMID: 8427984.

10. Coustan-Smith E, Kitanaka A, Pui CH, et al. Clinical relevance of BCL-2 overexpression in childhood acute lymphoblastic leukemia. Blood. 1996; 87:1140–1146. PMID: 8562940.

11. Signorelli P, Ghidoni R. Resveratrol as an anticancer nutrient: molecular basis, open questions and promises. J Nutr Biochem. 2005; 16:449–466. PMID: 16043028.

12. Lin MT, Yen ML, Lin CY, Kuo ML. Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of Src-dependent vascular endothelial cadherin tyrosine phosphorylation. Mol Pharmacol. 2003; 64:1029–1036. PMID: 14573751.

13. Seve M, Chimienti F, Devergnas S, et al. Resveratrol enhances UVA-induced DNA damage in HaCaT human keratinocytes. Med Chem. 2005; 1:629–633. PMID: 16787346.

14. Cullberg K, Foldager C, Lind M, Richelsen B, Pedersen S. Inhibitory effects of resveratrol on hypoxia-induced inflammation in 3T3-L1 adipocytes and macrophages. J Funct Foods. 2014; 7:171–179.

15. Fan GH, Wang ZM, Yang X, et al. Resveratrol inhibits oesophageal adenocarcinoma cell proliferation via AMP-activated protein kinase signaling. Asian Pac J Cancer Prev. 2014; 15:677–682. PMID: 24568477.

16. Lephart ED, Sommerfeldt JM, Andrus MB. Resveratrol: influences on gene expression in human skin. J Funct Foods. 2014; 10:377–384.

17. Gupta SC, Kannappan R, Reuter S, Kim JH, Aggarwal BB. Chemosensitization of tumors by resveratrol. Ann N Y Acad Sci. 2011; 1215:150–160. PMID: 21261654.

18. Dewson G, Kluck RM. Bcl-2 family-regulated apoptosis in health and disease. Cell Health Cytoskelet. 2010; 2:9–22.
19. Wojcik I, Szybka M, Golanska E, et al. Abnormalities of the P53, MDM2, BCL2 and BAX genes in acute leukemias. Neoplasma. 2004; 52:318–324.
20. Azimi A, Hagh MF, Talebi M, et al. Time-and concentration-dependent effects of resveratrol on miR 15a and miR16-1 expression and apoptosis in the CCRF-CEM acute lymphoblastic leukemia cell line. Asian Pac J Cancer Prev. 2015; 16:6463–6468. PMID: 26434860.
21. Tzifi F, Economopoulou C, Gourgiotis D, Ardavanis A, Papageorgiou S, Scorilas A. The role of BCL2 family of apoptosis regulator proteins in acute and chronic leukemias. Adv Hematol. 2012; 2012:524308. PMID: 21941553.

22. Winick NJ, Carroll WL, Hunger SP. Childhood leukemia-new advances and challenges. N Engl J Med. 2004; 351:601–603. PMID: 15295054.
23. Yin HT, Tian QZ, Guan L, Zhou Y, Huang XE, Zhang H. In vitro and in vivo evaluation of the antitumor efficiency of resveratrol against lung cancer. Asian Pac J Cancer Prev. 2013; 14:1703–1706. PMID: 23679260.

24. Jazirehi AR, Bonavida B. Resveratrol modifies the expression of apoptotic regulatory proteins and sensitizes non-Hodgkin's lymphoma and multiple myeloma cell lines to paclitaxel-induced apoptosis. Mol Cancer Ther. 2004; 3:71–84. PMID: 14749477.
25. Fulda S, Debatin KM. Sensitization for anticancer drug-induced apoptosis by the chemopreventive agent resveratrol. Oncogene. 2004; 23:6702–6711. PMID: 15273734.

26. Bhardwaj A, Sethi G, Vadhan-Raj S, et al. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007; 109:2293–2302. PMID: 17164350.
27. Fouad MA, Agha AM, Merzabani MM, Shouman SA. Resveratrol inhibits proliferation, angiogenesis and induces apoptosis in colon cancer cells: calorie restriction is the force to the cytotoxicity. Hum Exp Toxicol. 2013; 32:1067–1080. PMID: 23536519.
28. Dörrie J, Gerauer H, Wachter Y, Zunino SJ. Resveratrol induces extensive apoptosis by depolarizing mitochondrial membranes and activating caspase-9 in acute lymphoblastic leukemia cells. Cancer Res. 2001; 61:4731–4739. PMID: 11406544.
29. Estrov Z, Shishodia S, Faderl S, et al. Resveratrol blocks interleukin-1beta-induced activation of the nuclear transcription factor NF-kappaB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood. 2003; 102:987–995. PMID: 12689943.
30. Cecchinato V, Chiaramonte R, Nizzardo M, et al. Resveratrol-induced apoptosis in human T-cell acute lymphoblastic leukaemia MOLT-4 cells. Biochem Pharmacol. 2007; 74:1568–1574. PMID: 17868649.

Fig. 1
Evaluation of the effects of resveratrol (RES) and prednisolone (P) on the BAX expression level using quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR). CCRF-CEM cells were cultured and treated with resveratrol (15, 50, and 100 µM) and prednisolone (700 µM). BAX was examined in CCRF-CEM cells after 12, 24, and 48 hours of resveratrol and prednisolone treatment. CCRF-CEM cells without treatment were used as a control to evaluate the relative expression of BAX. The data are presented as mean±SD. The relative gene expression of the control was set as 1. (A) The BAX expression level in CCRF-CEM cells after 12 hours of treatment. (B) The BAX expression level in CCRF-CEM cells after 24 hours of treatment. (C) The BAX expression level in CCRF-CEM cells after 48 hours of treatment (a)P<0.05, b)P<0.01).
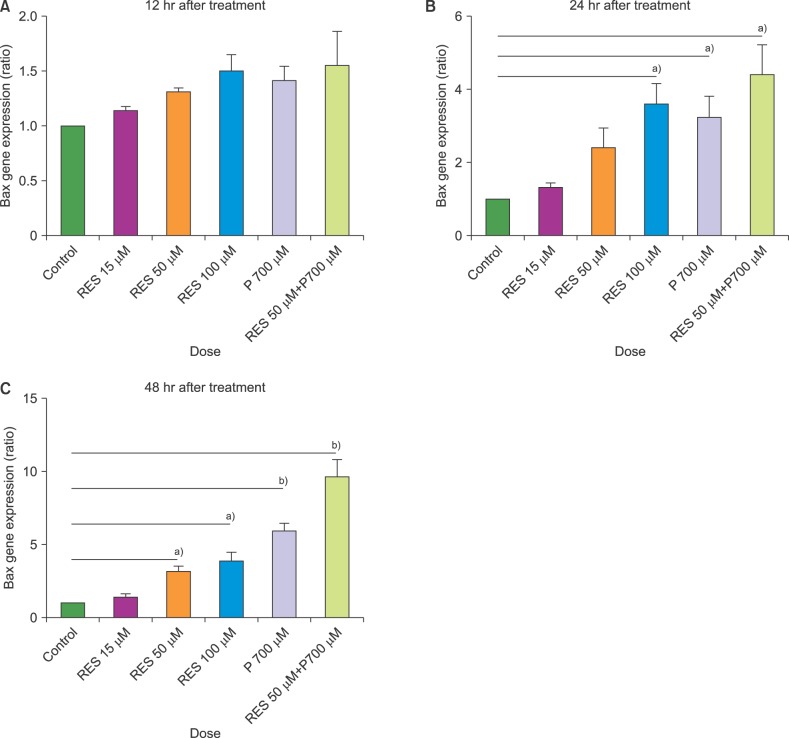
Fig. 2
Evaluation of the effects of resveratrol (RES) and prednisolone (P) on the BCL2 expression level using quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR). CCRF-CEM cells were cultured and treated with resveratrol (15, 50, and 100 µM) and prednisolone (700 µM). BCL2 was examined in CCRF-CEM cells after 12, 24, and 48 hours of resveratrol and prednisolone treatment. CCRF-CEM cells without treatment were used as a control to evaluate the relative expression of BCL2. The data are presented as mean±SD. The relative gene expression of the control was set as 1. (A) The BCL2 expression level in CCRF-CEM cells after 12 hours of treatment. (B) The BCL2 expression level in CCRF-CEM cells after 24 hours of treatment. (C) The BCL2 expression level in CCRF-CEM cells after 48 hours of treatment (a)P<0.05, b)P<0.01).
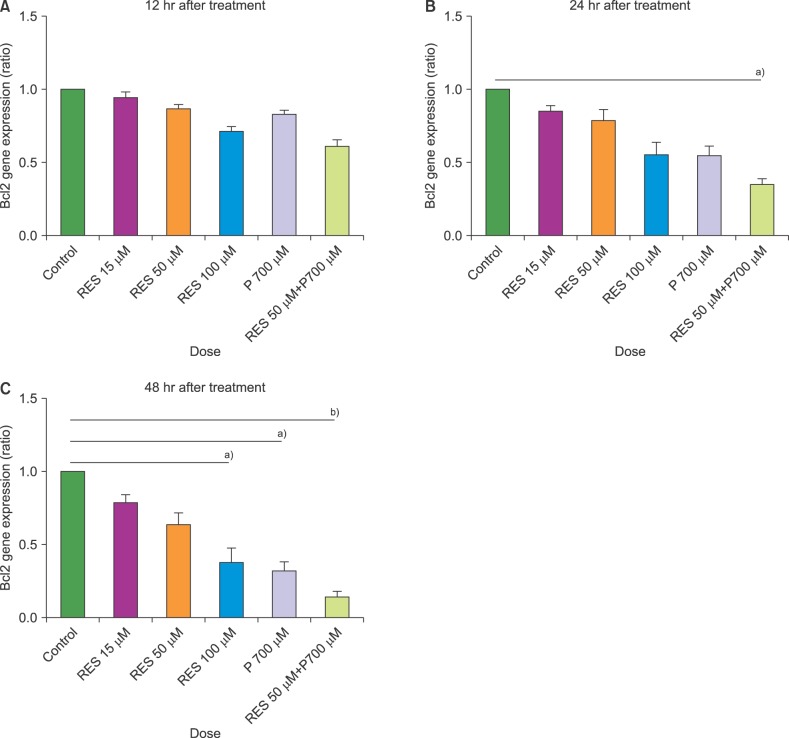
Fig. 3
Resveratrol (RES) and prednisolone (P) induced apoptosis in a dose- and time-dependent manner. CCRF-CEM cells were treated with resveratrol (15, 50, and 100 µM) and prednisolone (700 µM) and induced apoptosis in a dose-dependent manner after 24 hours (A) and 48 hours (B) (a)P<0.05, b)P<0.01).
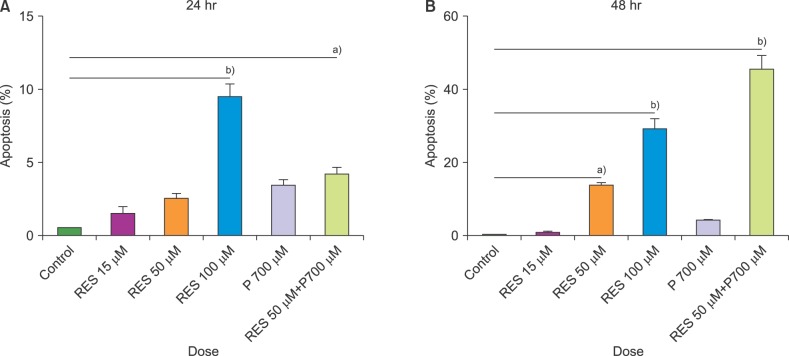
Fig. 4
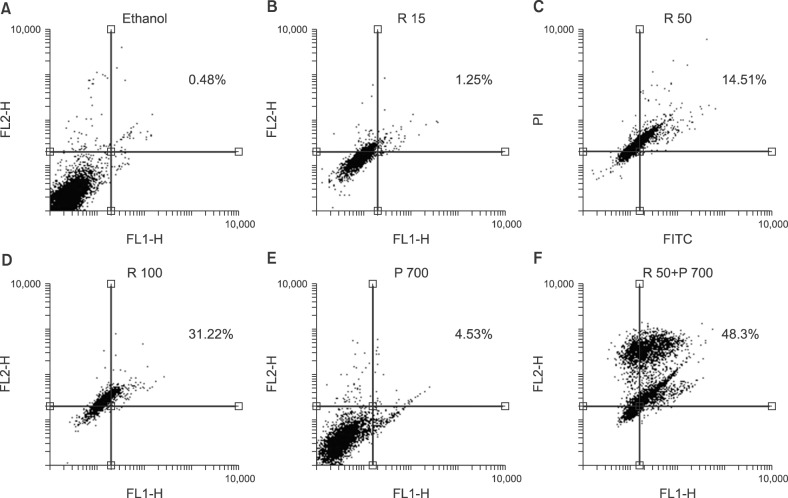
Representative data of double staining for annexin V-propidium iodide (PI) uptake from test groups and control by fluorescence activated cell sorting (FACS), after 24 hours of incubation. The percentage of PI-negative and annexin V-positive cells that were in early apoptosis, and PI positive and annexin V-positive cells that were dead or in end-stage apoptosis, are represented in each quadrant. (A) control, (B) 15 µM resveratrol (R), (C) 50 µM R, (D) 100 µM R, (E) 700 µM prednisolone (P), (F) 50 µM R+700 µM P. Ethanol, vehicle control.
Abbreviation: FITC, fluorescein isothiocyanate.
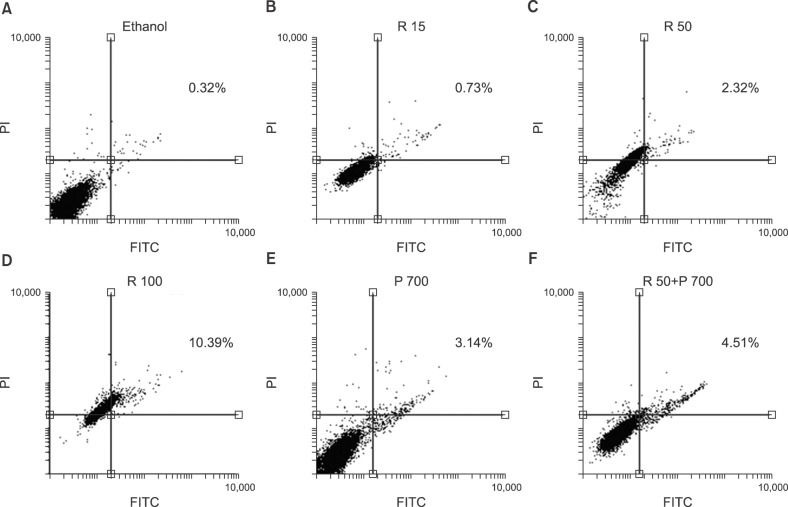
Fig. 5
Representative data of double staining for annexin V-propidium iodide (PI) uptake from test groups and control by fluorescence activated cell sorting (FACS), after 48 hours of incubation. The percentage of PI-negative and annexin V-positive cells that were in early apoptosis, and PI positive and annexin V-positive cells that were dead or in end-stage apoptosis, are represented in each quadrant. (A) control, (B) 15 µM resveratrol (R), (C) 50 µM R, (D) 100 µM R, (E) 700 µM prednisolone (P), (F) 50 µM R+700 µM P. Ethanol, vehicle control.
Abbreviation: FL1-H, height in the FL1 channel.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download