Abstract
Background
Additional cytogenetic aberrations are associated with disease progression in chronic myeloid leukemia (CML). This study was conducted to determine the type and frequency of these aberrations and their relationship with hematologic and molecular findings in the Middle East.
Methods
In this retrospective study, 134 well-established cases of CML were selected from 2010 to 2016. Their hematologic phase and type of fusion gene were determined. Finally, their karyotypes were analyzed and reported according to ISCN 2013.
Results
Patients had a mean age of 44 years. Twenty-two patients (16.4%) showed additional cytogenetic aberrations. Nine patients (6.7%) harbored a variant Philadelphia chromosome, and most were in the chronic phase. Seventeen patients (12.7%) had major and minor route abnormalities. There was a significant relationship between additional cytogenetic aberrations and major molecular response (P=0.032). Patient survival in the group with additional cytogenetic aberrations was significantly lower (49.7±11.1 mo) than that in the group without additional cytogenetic aberrations (77.3±3.1 mo) (P=0.031).
Go to : 
Chronic myeloid leukemia (CML) is a myeloid neoplasm characterized by a fusion gene, BCR-ABL, which leads to uncontrolled proliferation of cells of the myeloid series in all stages of differentiation [1]. The fusion gene is the result of a reciprocal translocation t (9;22) (q34;q11), known as the Philadelphia chromosome [2]. Although the Philadelphia chromosome is considered as a pioneer event in leukemogenesis, acquisition of additional cytogenetic aberrations may play a role in disease progression [3].
Recent studies showed that additional cytogenetic aberrations (ACA) result in CML progression from the chronic phase (CP) to the accelerated phase (AP) and blast crisis (BC) [145]. The most prevalent abnormalities in advanced stages of CML are trisomy 8, second Philadelphia chromosome, and isochromosome (17) (q10), which are major route abnormalities. All other ACAs are considered as minor route abnormalities [67].
No studies have examined the prevalence of additional cytogenetic aberrations in CML in the Middle East. Therefore, this study was conducted to determine the frequency and type of additional cytogenetic abnormalities in CML other than Philadelphia chromosome.
Go to : 
In this retrospective study, 134 patients diagnosed with CML were selected in a referral center in Shiraz (a Southern Iran Province) from 2010 to 2016 and their demographic data along with their hematologic and molecular (type of fusion gene detected by reverse transcription-polymerase chain reaction) findings were obtained from the medical records.
For cytogenetic evaluation, conventional synchronized culture was performed for 48 hours. Next, the samples were harvested and fixed by the addition of colcemid, hypotonic solution, and Carnoy's fixative. Finally, the slides were prepared by Giemsa staining. At least 15 fields were analyzed according to the International System for Human Cytogenetic Nomenclature 2013. Clonality was inferred when cells showed the same abnormal chromosomal abnormality. ACA was defined as the presence of any structural and numerical chromosomal abnormality other than t(9;22)(q34;q11) (detected by cytogenetic or molecular assays for cryptic abnormalities). A variant Philadelphia chromosome was defined as the presence of any translocation involving one or more chromosomes in addition to chromosomes 9 and 22.
Serial follow-up was performed by molecular assays and a major molecular response was defined according to European Leukemia Net. Achievement of 10%, 1%, and 0.1% on the BCR-ABL international scale during months 3, 6, and 12 after treatment initiation was considered as a major molecular response (MMR).
Go to : 
In this study, the mean age of patients was 44 years, ranging from 4 to 90 years and there were more males than females (male:female ratio of 1.8:1). A total of 109 of 134 patients were in the chronic phase (81.3%). The remaining 34 patients were in an accelerated phase (8.2%) and blast crisis (10.4%). Reverse transcription polymerase chain reaction was conducted for 89 patients (66.4%) and revealed 210 kDBCR-ABL fusions in 82 patients (61.2%) and 190 kDBCR-ABL fusions in 2 patients (1.5%). One patient (0.7%) showed both fusion genes, and 4 patients (3%) were negative for either of the 210 and 190 kD fusion genes. The remaining patients were undetermined for the types of fusion genes (33.6%).
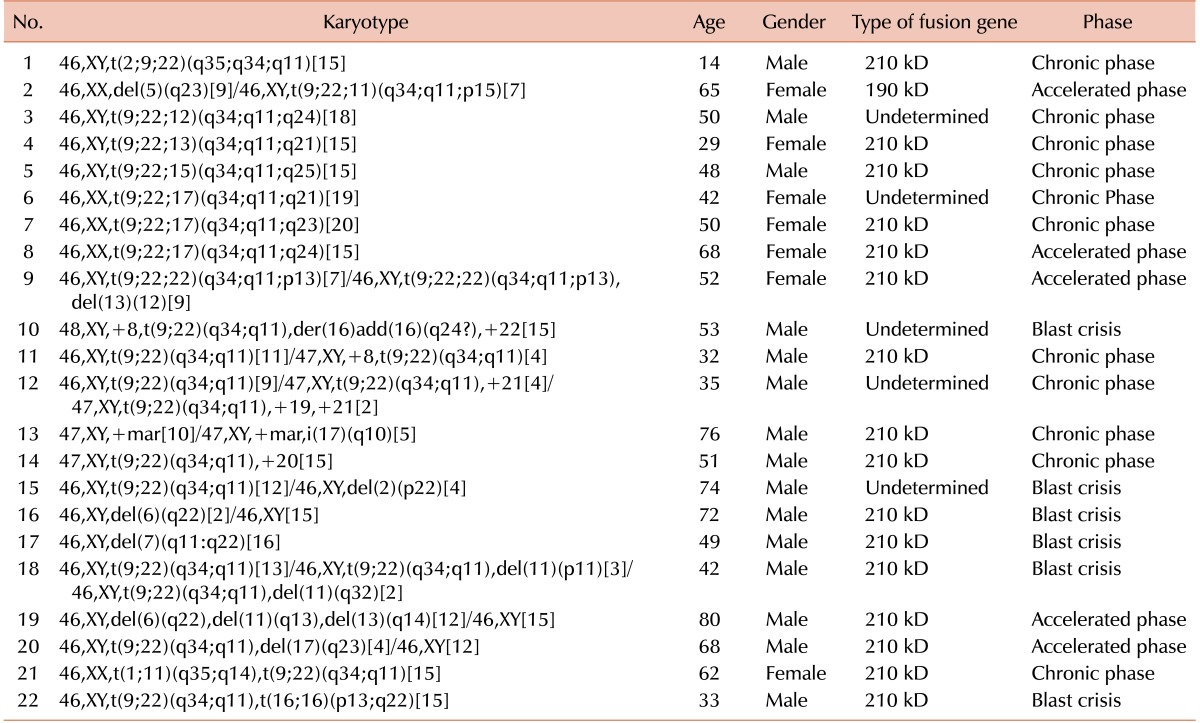
Twenty-two of 134 patients (16.4%) showed additional cytogenetic aberrations (Table 1). Nine patients (6.7%) had a variant Philadelphia chromosome translocation, which showed clustering to chromosome bands 2q35, 11p15 12q24, 13q21, 15q25, 17q21, 17q23, 17q11, and 22p13. Two of these 9 patients with a variant Philadelphia chromosome also had other chromosomal aberrations. Four and 13 patients among these 22 had major (3%) and minor (9.7%) route abnormalities, respectively. Four patients (2.9%) were negative for the Philadelphia chromosome based on cytogenetic analysis. One patient showed myelodysplastic syndrome in follow-up and progressed to acute myeloid leukemia. Balanced translocations were observed in 2 patients (1.5%).
There was a significant relationship between the variant Philadelphia chromosome and disease phase (P=0.038). Six patients (66.7%) with a Philadelphia variant chromosome were in the chronic phase. Only one patient (11.1%) with a Philadelphia variant chromosome as the sole chromosomal abnormality was in the accelerated phase. The other two patients (22.2%) with a variant Philadelphia chromosome who were in the accelerated phase had other additional cytogenetic aberrations. None were in the blast crisis. There was also a significant relationship between patient age and the presence of ACAs (P=0.016). The mean age of the patients with these aberrations was 52.3±17.3 years, which was approximately 10 years greater than the mean age of the patients without these aberrations (42.1±17.5). The relationship between disease phase and the presence of ACAs was significant (P<0.001). In CP, 10% of patients had ACAs, but this value was significantly higher in AP and BC, reaching 45.5% and 42.9%, respectively.
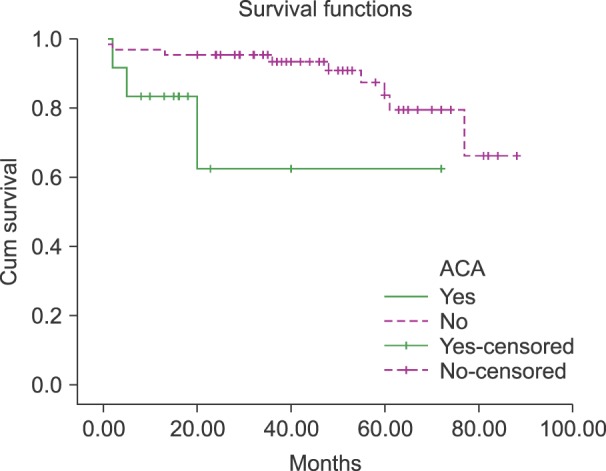
There was a significant relationship between ACA and MMR (P=0.032). Serial follow-up by molecular methods revealed that 72.5% of patients without ACA achieved MMR, but only 36% of subjects with ACA achieved an MMR. Survival analysis of 76 patients revealed that the survival of patients with ACA was significantly lower (49.7±11.1 mo) than the survival of those without ACA (77.3±3.1 mo) (P=0.031) (Fig. 1).
Go to : 
In this study, the patients had a mean age of 44 years, which is lower than the previously reported mean age of 65 years. As expected, more of the patients were male [8]. The overall frequency of ACAs was 16.4%, which was similar to the previously reported incidence [910].
A total of 6.7% of patients had a variant Philadelphia chromosome; the previously reported rate is 5–10% [1112]. Rearrangements involved chromosomes 2, 12, 13, 17, and 22. Chromosome 17 showed the greatest involvement. This chromosome was translocated recurrently to form the variant Philadelphia chromosome according to previous studies [7]. The impact of the variant Philadelphia chromosome on disease progression is controversial [13]. Some authors consider this chromosome as an indicator of poor prognosis, particularly before imatinib was used for treatment [11]. However, more recent studies revealed that patients with a variant Philadelphia chromosome had the same prognosis as those with a standard Philadelphia chromosome [14].
Major and minor route cytogenetic abnormalities were observed in 3% and 9.7% of patients, respectively, and were more common in advanced stages of CML. These findings support the pivotal role of these abnormalities in disease progression. Among major route abnormalities, trisomy 8 was the most common, followed by trisomy 19 and isochromosome (17)(q10), as previously reported. We did not detect an extra Philadelphia chromosome, +der(22)t(9;22) (q34;q11), a well-known major route abnormality. This abnormality was not detected in the GIMEMA Study of 559 patients with CML in Italy [15]. Minor route abnormalities were more common than major route aberrations and mainly included structural abnormalities and balanced translocation. In a recent study, the Randomized German CML Study IV, minor route abnormalities were more prevalent than major route abnormalities [6].
Interestingly, no patient with loss of chromosome Y was identified in this study as having a minor route abnormality. This finding indicates that loss of the Y chromosome is a consequence of aging and that the absence of this abnormality may be because of the lower mean age of the subjects. While some studies have demonstrated the significance of Y chromosome loss in CML [15], other studies found no relationship between this abnormality and disease, even after treatment and complete remission of Philadelphia-positive clones [6].
One limitation of this study was that follow-up could not be conducted because of a lack of access to the patients and the lack of an integrated system for patient registration.
The overall frequency of additional cytogenetic aberrations was similar that describe previously. Minor route abnormalities were more common than major route abnormalities and both were prevalent in advanced stages of disease, suggesting that their presence indicates disease progression. ACA resulted in poorer survival and less MMR.
Go to : 
ACKNOWLEDGMENTS
The authors would like to thank Shiraz University of Medical Sciences, Shiraz, Iran, Center for Development of Clinical Research of Namazee Hospital, and Ms. Marzieh Khojastehfar for help with statistical analysis.
Go to : 
Notes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
Go to : 
References
1. Hehlmann R, Hochhaus A, Baccarani M. European LeukemiaNet. Chronic myeloid leukaemia. Lancet. 2007; 370:342–350. PMID: 17662883.

3. Cortes J, O'Dwyer ME. Clonal evolution in chronic myelogenous leukemia. Hematol Oncol Clin North Am. 2004; 18:671–684. PMID: 15271399.

4. Melo JV, Barnes DJ. Chronic myeloid leukaemia as a model of disease evolution in human cancer. Nat Rev Cancer. 2007; 7:441–453. PMID: 17522713.

5. Skorski T. BCR/ABL, DNA damage and DNA repair: implications for new treatment concepts. Leuk Lymphoma. 2008; 49:610–614. PMID: 18398719.

6. Fabarius A, Leitner A, Hochhaus A, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011; 118:6760–6768. PMID: 22039253.

7. Johansson B, Fioretos T, Mitelman F. Cytogenetic and molecular genetic evolution of chronic myeloid leukemia. Acta Haematol. 2002; 107:76–94. PMID: 11919388.

8. Björk J, Albin M, Welinder H, et al. Are occupational, hobby, or lifestyle exposures associated with Philadelphia chromosome positive chronic myeloid leukaemia? Occup Environ Med. 2001; 58:722–727. PMID: 11600728.
9. Syed NN, Usman M, Adil S, Khurshid M. Additional chromosomal abnormalities in Philadelphia-positive chronic myeloid leukemia. Hematol Oncol Stem Cell Ther. 2008; 1:166–170. PMID: 20063547.

10. O'Dwyer ME, Gatter KM, Loriaux M, et al. Demonstration of Philadelphia chromosome negative abnormal clones in patients with chronic myelogenous leukemia during major cytogenetic responses induced by imatinib mesylate. Leukemia. 2003; 17:481–487. PMID: 12646934.
11. Albano F, Specchia G, Anelli L, et al. Genomic deletions on other chromosomes involved in variant t(9;22) chronic myeloid leukemia cases. Genes Chromosomes Cancer. 2003; 36:353–360. PMID: 12619159.

12. El-Zimaity MM, Kantarjian H, Talpaz M, et al. Results of imatinib mesylate therapy in chronic myelogenous leukaemia with variant Philadelphia chromosome. Br J Haematol. 2004; 125:187–195. PMID: 15059141.

13. Eyüpoğlu D, Bozkurt S, Haznedaroğlu İ, Büyükaşık Y, Güven D. The impact of variant Philadelphia chromosome translocations on the clinical course of chronic myeloid leukemia. Turk J Haematol. 2016; 33:60–65. PMID: 27020722.

14. Marzocchi G, Castagnetti F, Luatti S, et al. Variant Philadelphia translocations: molecular-cytogenetic characterization and prognostic influence on frontline imatinib therapy, a GIMEMA Working Party on CML analysis. Blood. 2011; 117:6793–6800. PMID: 21447834.

15. Luatti S, Castagnetti F, Marzocchi G, et al. Additional chromosomal abnormalities in Philadelphia-positive clone: adverse prognostic influence on frontline imatinib therapy: a GIMEMA Working Party on CML analysis. Blood. 2012; 120:761–767. PMID: 22692507.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download