TO THE EDITOR: Internists in intensive care units often encounter cases of acute respiratory distress syndrome (ARDS) that require mechanical ventilation. Such cases are difficult to manage and have a grim prognosis. Potential causes of ARDS include sepsis, trauma, pneumonia, infection, and uremia [1]. We describe a case of ARDS with associated eosinophilia that was diagnosed as a myeloid neoplasm with associated eosinophilia and PDGFRA gene rearrangement. Timely administration of imatinib mesylate saved the patient's life without the need for invasive ventilation.
A 35-year-old man presented to our emergency department after coughing for 7 days and experiencing respiratory distress for 1 day. He had no history of fever, nasal discharge, chest pain, hemoptysis, hematuria, or passage of worms in his stool. He denied any atopy, recent travel, or exposure to pets, birds, cotton, dust, or metal fumes. His vital signs were as follows: blood pressure, 110/70 mm hg; pulse rate, 112 beats/minute; and respiratory rate, 30 breaths/minute. Remarkably, a general examination showed the presence of pallor and the use of the accessory muscles of respiration. There was no lymphadenopathy, cyanosis, clubbing, pedal edema, or palpable purpura, and the jugular venous pressure was not elevated. Bilateral rhonchi and crackles were audible on chest auscultation. The liver (4 cm in size) and spleen (5 cm in size) were palpable on abdomen examination. The results of a cardiovascular and neurological examination were unremarkable. A complete blood count revealed an hemoglobin level of 8.3 g/dL; white cell count of 27.6×109/L; a differential count of 39% polymorphs, 11% lymphocytes, 2% monocytes, and 48% eosinophils (absolute eosinophil count, 13.2×109/L); a platelet count of 420×109/L; and an erythrocyte sedimentation rate of 24 mm/hour. Serum levels of lactate dehydrogenase (LDH) and uric acid were 1,000 U/L and 565 µmol/L, respectively, and renal and liver function were normal. The results of an arterial blood gas analysis were consistent with type-1 respiratory failure: pH, 7.38; partial pressure of oxygen, 67 mm hg; partial pressure of carbon dioxide, 35.5 mmHg; and oxygen saturation, 85%.
A chest X-ray revealed bilateral fluffy alveolar opacities (Fig. 1A). Cardiac size and contour were normal. Contrastenhanced computed tomography of the chest revealed symmetrical confluent airspace opacities in the bilateral central lung fields, suggestive of pulmonary edema (Fig. 2). Troponin I expression was negative, and pulmonary capillary wedge pressure (PCWP) measured by using a Swan-Ganz catheter was 5 mmHg. The serum procalcitonin level (0.3 µg/L) was normal, and blood cultures were sterile. Leptospira, mycoplasma, legionella, filariasis, and strongyloides stercoralis serology test results were negative, as were those for the legionella urine antigen, anti-nuclear antibody, and anti-neutrophil cytoplasmic antibody. Cysts/ova were not seen on stool examination. The serum immunoglobulin E level was 708 IU/mL, and nerve conduction was normal.
Owing to a possibility of Loeffler's pneumonia, diethylcarbamazine (300 mg in three divided doses) and a glucocorticoid (oral prednisone, 60 mg daily) were administered while awaiting a definitive diagnosis. The patient's symptoms did not improve and type-1 respiratory failure worsened; hence, bronchoscopy was performed. Bronchoalveolar lavage revealed an eosinophil-rich infiltrate, and histopathological examination of a transbronchial lung biopsy showed fibroblastic proliferation with formation of Masson bodies in the alveolar spaces (suggestive of organizing pneumonia), along with the presence of eosinophils in the interstitial spaces (Fig. 3A). Bone marrow examination revealed granulocytic hyperplasia and a marked increase in the number of eosinophils without any blasts. FIP1L1-PDGFRA mRNA was detected in the peripheral blood via reverse transcriptase polymerase chain reaction, whereas BCR-ABL1 mRNA was not.
The patient was diagnosed with a myeloid neoplasm associated with eosinophilia and PDGFRA gene rearrangement. Oral administration of imatinib (100 mg daily) led to a marked clinical, radiological, and hematological improvement within 1 week (Fig. 1B) and complete hematological remission (CHR) at 2 weeks (normalization of peripheral blood counts and no splenomegaly). He was subsequently discharged in stable condition. He is still undergoing regular follow-ups at the time of this write-up.
Eosinophilia is a relatively common finding in clinical practice and is often overlooked in the face of other clinical and biochemical features. Hypereosinophilic syndrome (HES) was initially defined by Chusid et al. [2] as (a) eosinophilia (>1.5×109/L eosinophils) for more than 6 months, (b) exclusion of secondary causes of eosinophilia such as drugs, allergies, and parasites, and (c) evidence of end organ damage. The first requirement was later removed to allow diagnosis of HES before permanent organ damage [3]. Owing to the recognition of recurrent cytogenetic and molecular abnormalities in patients with hypereosinophilia and because sustained eosinophilia can lead to end organ damage regardless of cause, eosinophilic disorders were reclassified by the World Health Organization in 2008 as (a) myeloid and lymphoid neoplasms associated with eosinophilia and abnormalities in the PDGFRA, PDGFRB, and FGFR1 genes, (b) chronic eosinophilia not otherwise specified, and (c) idiopathic HES. The term “HES” is now used to describe end organ damage secondary to hypereosinophilia of any etiology [4].
HES most commonly affects the hematological system (100%), cardiovascular system (58%), skin (56%), nervous system (54%), and pulmonary system (49%) [5]. Pulmonary involvement is a direct result of eosinophil-mediated damage or an indirect result of cardiac failure, pulmonary embolism, drug-related injuries, infection, or asthma [56]. The clinical presentation includes nocturnal coughing, wheezing, productive sputum, and dyspnea. Radiographic pulmonary abnormalities (14–28% of cases) include nodules, interlobular septal thickening, ground-glass opacities, pleural effusion, focal or diffuse infiltrates, atelectasis, fibrosis, and on rare occasions, hilar adenopathy and pulmonary hypertension [6]. Occurrence of ARDS in HES is extremely rare, with only a few case reports [67]. In a study by Dulohery et al. [6] of 49 patients with HES, symptomatic pulmonary involvement, radiographic abnormalities (parenchymal infiltrates, pleural effusion, lymphadenopathy, and emboli), and asthma were observed in 63%, 43%, and 27% of the patients, respectively; none of the cases involved ARDS. The most commonly reported radiographic findings for HES are ground glass opacities and patchy consolidation.
Imatinib (100–400 mg daily) is the standard treatment for myeloid/lymphoid neoplasms with PDGFRA gene rearrangements, and almost all patients with this disease achieve CHR and a 3-log reduction in PDGFRA transcript levels within 1 month and 1 year of therapy, respectively. Primary and acquired imatinib resistance is unusual. Relapses after imatinib discontinuation have been documented, but molecular remission can be successfully achieved following imatinib re-initiation [8]. The absence of troponin-I expression and a normal PCWP ruled out cardiogenic pulmonary edema secondary to heart involvement in our case.
The present case highlights two important points: HES is an important reversible etiology of ARDS, and eosinophilia in the setting of ARDS should never be overlooked. The presence of organomegaly and an elevated LDH level may provide early clues as to the underlying myeloproliferative process. FIP1L1-PDGFRA transcripts must be identified as early as possible in HES cases, as treatment with imatinib may aid patients without the need for mechanical ventilation.
Figures and Tables
Fig. 1
(A) Chest X-ray (posteroanterior view) showing diffuse alveolar opacities with a normal cardiac shadow. (B) Chest X-ray 1 week after treatment with imatinib mesylate showing complete resolution of the infiltrates.
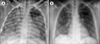
Fig. 2
(A, B) Computed tomography scans showing confluent symmetrical central airspace shadows indicating pulmonary edema.
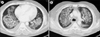
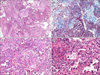
Fig. 3
Photomicrographs showing (A) intra-alveolar Masson bodies indicating an organizing pneumonia pattern (hematoxylin and eosin, ×200), (B) Masson bodies (Masson's trichrome stain, ×200), (C) a few alveoli with fibrin balls (hematoxylin and eosin, ×200), and (D) an interstitial eosinophilic infiltrate (hematoxylin and eosin, ×400).
References
1. Fowler AA, Hamman RF, Good JT, et al. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med. 1983; 98:593–597.

2. Chusid MJ, Dale DC, West BC, Wolff SM. The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine (Baltimore). 1975; 54:1–27.
4. Gotlib J. World Health Organization-defined eosinophilic disorders: 2014 update on diagnosis, risk stratification, and management. Am J Hematol. 2014; 89:325–337.

5. Gotlib J, Cools J, Malone JM 3rd, Schrier SL, Gilliland DG, Coutré SE. The FIP1L1-PDGFRalpha fusion tyrosine kinase in hypereosinophilic syndrome and chronic eosinophilic leukemia: implications for diagnosis, classification, and management. Blood. 2004; 103:2879–2891.

6. Dulohery MM, Patel RR, Schneider F, Ryu JH. Lung involvement in hypereosinophilic syndromes. Respir Med. 2011; 105:114–121.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download