INTRODUCTION
Primary central nervous system lymphoma (PCNSL) is among the most aggressive variants of extranodal non-Hodgkin lymphoma that involves the brain, cranial nerves, meninges, eyes, or spinal cord. It is is known to be sensitive to both radiotherapy and chemotherapy. However, this sensitivity does not guarantee high yields of disease control or cure [
1]. Although new therapeutic strategies have improved treatment outcomes, they are rarely curative. PCNSL tends to recur and carry a dismal prognosis in most patients.
Several prognostic factors influence patient survival [
234567]. Of these, the most consistent variables are patient age and performance status. To date, 3 Western prognostic models have been proposed. The first model was proposed by the International Extranodal Lymphoma Study Group (IELSG) in 2003 and is composed of 5 prognostic variables: age more than 60 years, Eastern Cooperative Oncology Group (ECOG) performance status more than 1, elevated level of serum lactate dehydrogenase (LDH), high cerebrospinal (CSF) protein concentration, and involvement of the deep regions of the brain such as the periventricular regions, basal ganglia, brainstem, and/or cerebellum [
8]. However, this model has some limitations such as the relatively small number of patients (N=105) and short follow-up period (median, 24 mo). Another European organization devised a 4-point scoring system using age, ECOG performance status, and multifocal disease [
9]. However, the Nottingham/Barcelona prediction scoring system also has its own drawbacks originating from the small number of patients (N=77), discrimination issues between stratified risk groups (score 1 group vs. score 2 group), and use of old-fashioned chemotherapeutic regimens. Finally, the Memorial Sloan-Kettering Cancer Center (MSKCC) proposed a simple but statistically powerful model using only age and performance status in 2006 [
6]. However, this model may have an inherent selection bias because retrospective data were collected from a single large institution.
Additionally, some studies reviewed the natural course and treatment outcomes of Asian patients [
10111213]. However, their findings were also derived from retrospective, heterogeneous cohorts. We reviewed the clinical characteristics and survival outcomes of patients with pathologically diagnosed PCNSL with pure diffuse large B-cell histology. Moreover, prior models were validated, and we developed a new model for pretreatment risk stratification.
MATERIALS AND METHODS
Patients
Seventy-seven patients with PCNSL diagnosed at least 18 months before enrollment between October 1998 and March 2012 in Asan Medical Center were enrolled in this study. Patients who met the following inclusion criteria were recruited to maximize homogeneity of the study cohort: a) histologically proven diffuse large B-cell lymphoma of PCNSL; b) immune competent at diagnosis without any evidence of human immunodeficiency virus infection or other congenital immunodeficiency; c) disease localized only in the brain, cranial nerves, meninges, eyes (excluding the orbit or ocular adnexa), or spinal cord; and d) no prior history of malignancy.
Patient information including age, gender, ECOG performance status, and treatment modalities was retrieved from medical records. All patients were pathologically confirmed to have primary CNS diffuse large B-cell lymphoma by experienced pathologists in our institution based on the World Health Organization criteria [
14]. Radiologic reviews were performed using Picture Archiving and Communication System. Multifocal disease was defined as more than one lesion found in radiologic evaluation, and deep regions of the brain were defined as the periventricular regions, basal ganglia, brainstem, and/or cerebellum.
Staging and response evaluation
Staging evaluations involved a physical examination, including slit-lamp assessment; contrast-enhanced magnetic resonance imaging (MRI) of the brain; computed tomography (CT) of the thorax, abdomen, and pelvis; lumbar puncture and CSF cytology and protein measurements (normal value: 15−45 mg/dL) if not contraindicated; bilateral bone marrow (BM) aspiration and biopsy; HIV serology test; complete blood cell count with differential count; liver and kidney function tests; serum LDH (normal level: 140−250 IU/L) level; and β2-microglobulin (β2-MG, normal level: 1.0−2.4 mg/L) level.
Treatment responses were assessed according to the International Group for P3CNSL criteria [
15]. Routine follow-up imaging (MRI or CT) and ophthalmologic examinations were performed every 3 months for the first 2 years, every 6 months for the next 3 years, and every year thereafter or whenever clinically indicated.
IELSG, Nottingham/Barcelona, and MSKCC prognostic models
The IELSG prognostic scoring system uses 5 unfavorable variables, namely, age more than 60 years, performance status more than 1, elevated LDH level, high CSF protein concentration, and involvement of deep regions of brain. And each value is assigned a score of 1 that are then added to yield a final score (0 to 1 score group; 2 to 3 score group; 4 to 5 score group) [
8]. The Nottingham/Barcelona model incorporates 3 variables, namely, age 60 years or older, ECOG performance status more than 1, and multifocal disease. Giving 1 point for each adverse variable, the risk groups are divided into 4 types (0, 1, 2, and 3) [
9]. Meanwhile, the MSKCC prognostic score is a simplified index that uses only age and Karnofsky Performance Status Scale (KPS scale) and has 3 prognostic classes: class 1 (age <50 yr), class 2 (age ≥50 yr or KPS ≥70%), and class 3 (age ≥50 yr and KPS <70%) [
6].
Statistical analysis
Overall survival (OS) was defined as the time from the date of diagnosis to death from any cause or to the date of last follow-up. Event-free survival (EFS) was calculated from the date of diagnosis to that of relapse, disease progression, death from any cause, or last follow-up. OS and EFS were estimated using the Kaplan-Meier method and were compared using the log-rank test. Multivariate analyses were carried out using the Cox proportional hazards model. A two-sided P-value of less than 0.05 was considered statistically significant. All analyses were performed using SPSS statistical software package, version 19.0 (SPSS Inc., Chicago, IL, USA).
DISCUSSION
If untreated, PCNSL is rapidly fatal. WBRT had been widely used as the first-line treatment for patients with PCNSL and has a median survival of approximately 1 year [
16]. WBRT has been attempted to be combined with chemotherapy, but high rates of delayed neurotoxicities became problematic, particularly in vulnerable elderly patients. The introduction of high-dose MTX-based chemotherapeutic regimen in the 1990s improved the response rate and prolonged patient survival. Currently, the median survival of patients with PCNSL treated with MTX-based chemotherapy ranges from 14 months to 5 years [
17]. High-dose chemotherapy followed by ASCT is now widely accepted as an alternative treatment modality in selected patients and can be used as first-line as well as salvage treatment. We recently reported good response rates, favorable tolerance profiles, and a 2-year survival rate of 88.9% from induction chemotherapy (5 cycles of high-dose MTX and 2 cycles of high-dose cytarabine) followed by conditioning with BUCYE (busulfan, cyclophosphamide, and etoposide) and then ASCT [
18].
The most widely accepted prognostic markers for patients with PCNSL are age and performance status [
1]. In our previous report, we found that age was the strongest prognostic factor for survival irrespective of treatment scheme. However, that study focused on relapse patterns of PCNSL, and the study population was heterogeneous in nature [
10]. In the present study, we enrolled only patients who met the inclusion criteria (importantly, only those with diffuse large B-cell histology) to maximize homogeneity of a cohort. Moreover, age over 65 years, multifocal lesion, and high CSF protein concentrations were found to be independent predictors for poor prognosis. Interestingly, chemotherapy followed by ASCT was associated with OS and EFS in univariate analysis but not in multivariate analysis. Thus, we performed a subgroup analysis of patients younger than 65 years old who received chemotherapy as first-line treatment to determine if chemotherapy followed by ASCT influences prognosis. However, no statistically meaningful survival differences were found between the 2 groups. This result might be due to the relatively small number of patients and different induction or conditioning regimens applied to the patients in the ASCT group.
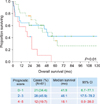
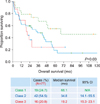
Prognostic models that can help clinicians estimate patient prognosis, determine optimal treatment modalities, and facilitate clinical trials have been proposed by many researchers. However, the application of these models is limited by their complexity to use, weak cross-validity, and inherent heterogeneity among study populations. We found that the Nottingham/Barcelona and MSKCC models predicted our patients' survival, but the IELSG model did not. In particular, the MSKCC model, which used recursive partitioning analysis technique to secure homogeneity, worked adequately to stratify the risk groups. However, we have to keep in mind that the Nottingham/Barcelona model was based on old-fashioned chemotherapeutic regimens, and the MSKCC model has an issue of selection bias.
Our newly designed model is based on 3 identified variables, namely, age, numbers of tumor, and CSF protein level. Unlike other models, the performance status was not a predictive factor for survival in our study. In our cohort, the proportion of patients with good performance status (ECOG PS <2 or KPS ≥70) was relatively higher (74.0%) than that of the Western studies (IELSG, 31%; Nottingham/Barcelona, 36%; and MSKCC, 55%). This discrepancy may be attributed to the overestimation of clinician-reported performance status while collecting retrospective data and the sociocultural tendency to hide the severity of cancer-related symptoms. We found that the cut-off value of age was 65 years, which is an also important factor when deciding on stem cell transplantation for patients with lymphoma. Measuring the CSF protein level is sometimes contraindicated in patients suspected with increased intracranial pressure; however, most patients (61/77, 79.2%) underwent spinal tapping without compromising our scoring system. Involvement of the deep regions of the brain was considered important in the IELSG model, but subsequent studies, including our study, found no correlation between deep structure involvement and prognosis. In view of risk group stratifications, the Nottingham/Barcelona model and our model subdivide high-risk groups into “higher-” and “highest-” risk groups. In terms of homogeneity and treatment regimens, our model appears to be a better tool for predicting prognosis between the groups. As such, the AMC model can be an alternative tool for predicting prognosis in patients with primary CNS diffuse large B-cell lymphoma.
To identify other readily available predictors, we added serum β
2-microglobulin and variables such as absolute lymphocyte count [
19], neutrophil-to-lymphocyte ratio [
20], and platelet-to-lymphocyte ratio [
21] into our analyses. Contrary to our expectations, no factors were identified to be significantly associated with prognosis (data not shown). Further investigations on these variables are needed to confirm their usefulness.
The major limitations of our study are the possibility of selection bias due to the single-center, retrospective study design and lack of external validation. Our findings necessitate further validations by larger cohort, multicenter prospective clinical trials.
In our study, advanced age, multifocal lesions, and high CSF protein concentration were identified as adverse prognostic variables. In PCNSL patients with pure diffuse large B-cell histology, new model can be useful in estimating patient prognosis.






 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download