TO THE EDITOR: Interdigitating dendritic cell sarcoma (IDCS) is a very rare disease entity characterized by neoplastic proliferation of spindle-shaped cells with phenotypic features similar to those of interdigitating dendritic cell (IDC). To date, only about 100 cases of IDCS have been reported in English literature [1], and because of its rarity, the pathophysiology is not fully understood. Interestingly, IDCS has been reported to be associated with other malignancies, in particular lymphoid malignancies [23]. Traditionally, IDC has been recognized as a myeloid lineage due to its functional similarities to macrophages. Therefore, IDCS frequently occurring in patients with B-cell lymphoid malignancies is difficult to explain through the usual hematopoietic process, which is uni-directional and has irreversible lineage commitment. In this paper, we present a rare case of a patient who was diagnosed as NK/T cell lymphoma 19 months after achieving complete remission of IDCS with literature review.
A 77-year-old female presented to our institution with palpable left lower quadrant abdominal mass in September 2015. Abdominal computed tomography (CT) scan showed a soft tissue mass near the left external iliac nodal area (Fig. 1A). Subsequent 18fluorodeoxyglucose-positron emission tomography (18 FDG PET)-CT showed high FDG uptake only on the same lesion (Fig. 1B). A core needle biopsy of the lesion revealed atypical T-cell proliferation with angiodestructive pattern (Fig. 2). Neoplastic cells were strongly positive for CD4, granzyme B, CD3, and CD8 but negative for CD20, CD1a, S-100, and CD56a. Fluorescent in situ hybridization of the biopsy specimen was positive for Epstein-Barr virus (EBV). These findings were consistent with NK/T cell lymphoma. Considering limited lesion, old age, and poor general condition of the patient, we performed radiation therapy for the abdominal mass. However, 1 month after completion of treatment, she complained of aggravated general condition with a febrile sensation. On sequentially performed PET-CT (Fig. 1C, D), disseminated FDG-avid masses were identified on the nasopharynx, lymph nodes in multiple areas, spleen, and both lungs, whereas the radiated abdominal mass regressed. Biopsy on the nasopharynx also revealed NK/T cell lymphoma. Despite Aspa-Met-Dex chemotherapy (dexamethasone 40 mg on days 1–4; high-dose methotrexate 3 g/m2 on day 1; and L-asparaginase 6,000 U/m2 on days 2, 4, 6, and 8 every 21 days), her condition drastically deteriorated only after the first cycle of chemotherapy, and she died of disease progression and pneumonia. Interestingly, 2 years earlier, she was diagnosed with IDCS after pathologic evaluation of newly developed cervical lymphadenopathy (Fig. 3). At that time, biopsy of the cervical lymph node showed S-100 and CD68 staining spindle- to ovoid-shaped cells intermingled with reactive T lymphocytes that are distinctively different from the pathologic finding of abdominal mass in this time. She received 6 cycles of ABVD chemotherapy (doxorubicin 25 mg/m2, bleomycin 10 mg/m2, vinblastine 6 mg/m2, and dacarbazine 375 mg/m2 on days 1 and 15 every 28 days) for IDCS and achieved complete remission. She has remained well for 19 months (Fig. 2B).
The World Health Organization classifies dendritic cells (DCs) into 4 types: follicular, interdigitating, Langerhans cell, and fibroblastic cells. DC neoplasms are classified based on those 4 types of normal counterpart. Among them, IDCs are primarily distributed in the thymus or T-cell zones of lymphoid organs where they are responsible for presenting various antigens on the cell surface to the T-cells of the immune system. As mentioned earlier, IDCS cases have been frequently reported to be accompanied by synchronous or metachronous lymphoid malignancies. To elucidate this unique phenomenon, a few putative mechanisms have been proposed. The cross-lineage “trans-differentiation” theory is among the most convincing concepts. In particular, several recent studies demonstrated a clonal relationship between a few indolent B cell lymphoma and IDCS that occurred synchronously or metachronously in the same patient by showing the presence of identical clonal immunoglobulin (Ig) gene rearrangement or showing that the Ig gene harbors the same molecular or cytogenetic abnormalities in 2 different malignancies [45]. Moreover, some studies supported the lineage plasticity of DC by showing an inheritance of B-cell or T-cell genotype in patients with histiocytic/DC malignancies [6]. Although we did not perform comparative molecular study including assessment of T-cell receptor gene rearrangement, the 2 different hematologic malignant cells in our case are less likely clonally related with each other because NK/T cell usually does not rearrange T-cell receptor gene [7] and most previously reported secondary IDCS cases occurred after or concurrently with lymphoid malignancies. Shared etiologic factors such as common genetic abnormalities or viral etiology between NK/T cell lymphoma and IDCS can be considered as another potential mechanism. In NK/T cell lymphoma, EBV has been recognized as a principal pathogenetic factor. By contrast, viral etiology such as EBV has not been supported for IDCS, although follicular dendritic cell sarcoma (FDCS) is known to be associated with EBV infection [1]. Recently, B-RAF V600E mutation has been shown to be associated with a subset of histiocytic/DC tumors. In particular, O'Malley et al. [8] reported a case of IDCS that harbored B-RAF V600E mutation and was combined with B-cell lymphoma. However, considering the low incidence of B-RAF mutation in NK/T cell lymphoma, the probability of the 2 hematologic malignancies being related with B-RAF mutation is low [9]. Although the above theories cannot be applied to our case, 1 potential mechanism is helpful to explain this unique phenomenon. Because DCs play a crucial role in introducing tumor antigens to immune cells, particularly naïve T-cells, a diminished immune response of DCs caused by malignant transformation may contribute to the occurrence of secondary malignancy [10]. Considering the temporal order of 2 malignancies in our case, this theory is more convincing. Also, IDCS being more frequently associated with secondary malignancies than other subtypes of DC/histiocytic tumors support this theory. FDCS, which are mainly involved in humoral immunity, are frequently associated with autoimmune disease rather than secondary malignancy. By contrast, IDCS are mainly involved in cellular immunity, which is required in antitumor immunity.
To the best of our knowledge, this is the first case in which IDCS and metachronous NK/T cell lymphoma were confirmed in 1 patient. Our case provides additional evidence supporting that patients with IDCS tend to have concurrent or metachronous malignancies. As such, more thorough cancer surveillance is necessary in patients with IDCS even after complete remission.
References
1. Saygin C, Uzunaslan D, Ozguroglu M, Senocak M, Tuzuner N. Dendritic cell sarcoma: a pooled analysis including 462 cases with presentation of our case series. Crit Rev Oncol Hematol. 2013; 88:253–271. PMID: 23755890.

2. Vasef MA, Zaatari GS, Chan WC, Sun NC, Weiss LM, Brynes RK. Dendritic cell tumors associated with low-grade B-cell malignancies. Report of three cases. Am J Clin Pathol. 1995; 104:696–701. PMID: 8526215.
3. Cossu A, Deiana A, Lissia A, et al. Synchronous interdigitating dendritic cell sarcoma and B-cell small lymphocytic lymphoma in a lymph node. Arch Pathol Lab Med. 2006; 130:544–547. PMID: 16594749.

4. Feldman AL, Arber DA, Pittaluga S, et al. Clonally related follicular lymphomas and histiocytic/dendritic cell sarcomas: evidence for transdifferentiation of the follicular lymphoma clone. Blood. 2008; 111:5433–5439. PMID: 18272816.

5. Shao H, Xi L, Raffeld M, et al. Clonally related histiocytic/dendritic cell sarcoma and chronic lymphocytic leukemia/small lymphocytic lymphoma: a study of seven cases. Mod Pathol. 2011; 24:1421–1432. PMID: 21666687.

6. Chen W, Lau SK, Fong D, et al. High frequency of clonal immunoglobulin receptor gene rearrangements in sporadic histiocytic/ dendritic cell sarcomas. Am J Surg Pathol. 2009; 33:863–873. PMID: 19145200.
7. Miyata-Takata T, Takata K, Yamanouchi S, et al. Detection of T-cell receptor γ gene rearrangement in paraffin-embedded T or natural killer/T-cell lymphoma samples using the BIOMED-2 protocol. Leuk Lymphoma. 2014; 55:2161–2164. PMID: 24308432.

8. O'Malley DP, Agrawal R, Grimm KE, et al. Evidence of BRAF V600E in indeterminate cell tumor and interdigitating dendritic cell sarcoma. Ann Diagn Pathol. 2015; 19:113–116. PMID: 25787243.
9. Ma C, Zhang X, Zhao Y, Wang G, Zhang M. Detection and significance of BRAF gene in mature T/NK cell lymphoma. Zhonghua Zhong Liu Za Zhi. 2015; 37:816–822. PMID: 26887510.
10. Pokuri VK, Merzianu M, Gandhi S, Baqai J, Loree TR, Bhat S. Interdigitating dendritic cell sarcoma. J Natl Compr Canc Netw. 2015; 13:128–132. PMID: 25691604.

Fig. 1
Abdominal CT image shows an approximately 4.5 cm-sized low-density mass-like lesion at the Lt external iliac nodal region (A). On sequentially performed PET-CT, increased FDG uptake was identified on the same lesion (B). Two months after diagnosis of NK/T cell lymphoma, follow-up PET-CT revealed extensive multiple hypermetabolic mass on areas including both lungs, spleen, and nasal cavity (C, D).
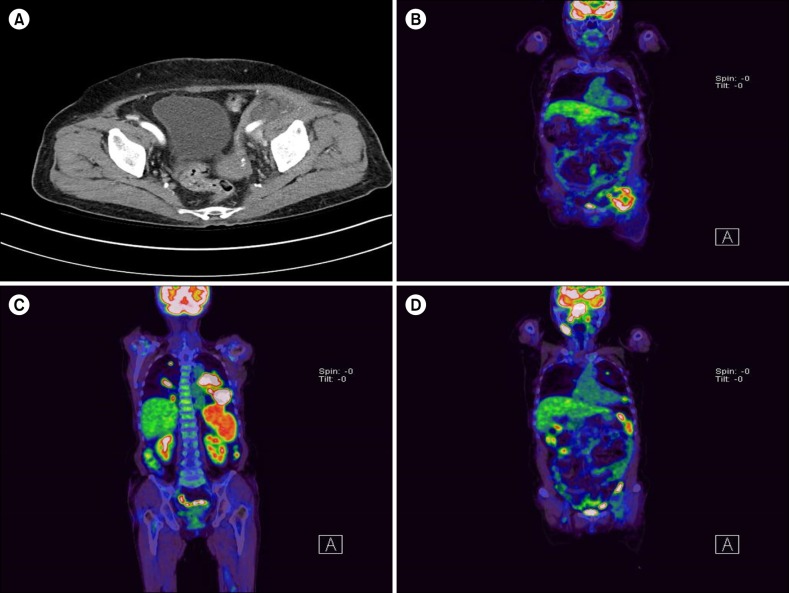
Fig. 2
Histological findings of soft tissue show atypical T-cell proliferation with angiocentric pattern in (A) hematoxylin-eosin stain (×200), and (B) CD3 immunohistochemical (IHC) stain. Additional IHC staining of cancer cells shows positivity for granzyme (C), CD68 (D), and EBV (F) but negativity for CD1a (E). These findings are consistent with nasal type extranodal NK/T cell lymphoma.
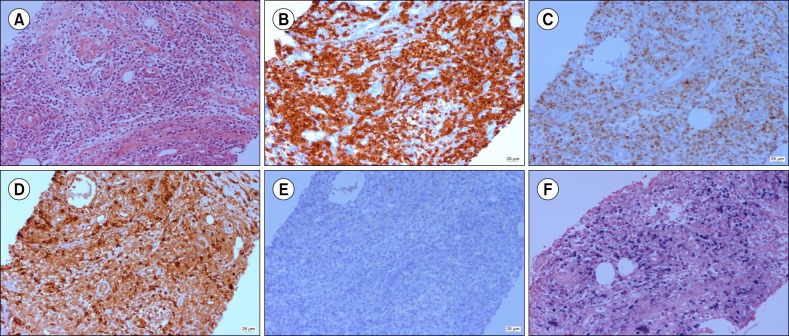
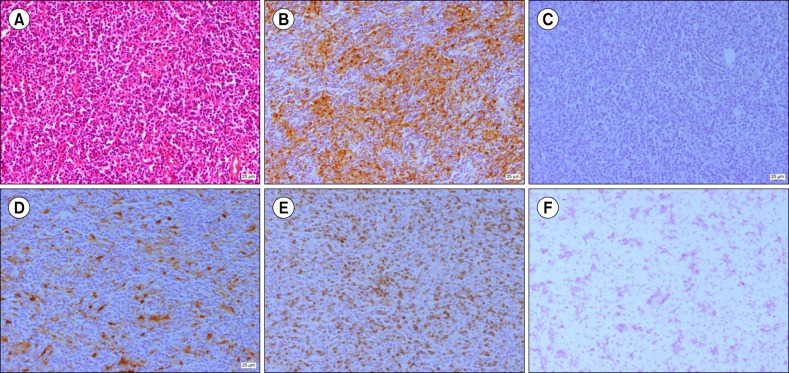
Fig. 3
Histological findings of cervical lymph node show spindle- to ovoid-shaped cells arranged intermingled with reactive lymphocytes in (A) hematoxylin-eosin stain (×200). IHC staining of cancer cells shows positivity for CD68 (B) and S100 (D) but negativity for CD1a (C) and EBV (F). Intermingled T-lymphocytes within IDCS show positivity for CD3 (E).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download