Abstract
Background
CD5-positive diffuse large B-cell lymphoma (CD5+ DLBCL) accounts for 5–10% of DLBCL cases and has poor patient outcomes. However, most studies on CD5+ DLBCL were performed in Japanese patients and only few data are available for Korean population. In this study, we investigated the clinical characteristics and prognostic impact of CD5 expression in Korean patients with bone marrow (BM) involvement of DLBCL.
Methods
Patients who were initially diagnosed with BM involvement of de novo DLBCL from 2005 to 2013 were included. Clinicopathological features and outcomes of patients were compared between CD5+ and CD5 negative (CD5−) DLBCL.
Results
Among a total of 57 patients, the number of patients with CD5+ and CD5− DLBCL were 13 and 44, respectively. Clinical and laboratory features of CD5+ DLBCL were not significantly different from those of CD5− DLBCL. The 3-year overall survival (OS) rates for CD5+ and CD5− DLBCL were 20.2% and 59.0%, respectively (P=0.031), and 3-year progression-free survival (PFS) rates for CD5+ and CD5− DLBCL were 23.1% and 50.1%, respectively (P=0.055).
Diffuse large B-cell lymphoma (DLBCL) is the most common subcategory of non-Hodgkin's lymphoma (NHL), accounting for 25–30% of NHL in Western countries [1] and approximately 43% in Korea [2]. Patients with DLBCL have highly variable clinical outcomes, representing a heterogeneous group of tumors with different morphology, histology, clinical features, responses to treatment, and prognosis.
The International Prognostic Index (IPI) is the classical clinical tool for predicting outcomes in patients with aggressive NHL including DLBCL [3]. Although the IPI is an important prognostic factor for DLBCL, the five components used for assessing the IPI provide limited information on biological features. The CD5-positive (CD5+) DLBCL, as a distinct subgroup that accounts for 5–10% of all DLBCL, has been reported to be associated with elderly onset, female gender, frequent involvement of extranodal sites, and inferior survival [4]. Recently, several studies reported that CD5 expression is a prognostic factor for poor outcome in DLBCL, even with rituximab-containing therapy [5678910]. However, most studies on CD5+ DLBCL were performed in Japan [5678]. In this study, we evaluated the clinicopathological characteristics and prognostic impact of CD5 expression in Korean patients with initial bone marrow (BM) involvement of DLBCL.
Patients who were initially diagnosed with BM involvement of de novo DLBCL at Samsung Medical Center (a 1,961-bed referral hospital in Seoul, Korea) between January 2005 and April 2013 were retrospectively included. DLBCL was diagnosed based on the World Health Organization classification. Patients who were not evaluated for CD5 expression of DLBCL or who had a previous history of other lymphoproliferative disorders such as chronic lymphocytic leukemia or mantle cell lymphoma were excluded. Clinical and laboratory information of patients was retrieved from electronic medical records. This study was approved by the Institutional Review Board of our institute.
CD5 antigen expression was examined by flow cytometric analysis and/or immunohistochemistry. Flow cytometric analysis was performed with monoclonal antibodies for CD5, CD10, CD19, CD20, CD22, CD23, FMC7, nuclear TdT, and kappa (κ) and/or lambda (λ) immunoglobulin using the three-laser FACSCanto II flow cytometer (Becton-Dickinson, San Jose, CA, USA). Data were acquired and analyzed with the BD FACSDiva software (Becton-Dickinson). Immunohistochemical analysis was carried out using monoclonal antibodies against CD5, CD10, BCL2, BCL6, Ki-67, MUM1, and/or cyclin D1. To classify the samples into immunohistochemically defined germinal center B-cell like (GCB) or non-GCB phenotypes, we used an algorithm previously described by Hans et al. [11].
Conventional cytogenetic studies were performed on heparinized BM samples from study participants. Each sample was cultured for 24 and/or 72 hours of lipopolysaccharide stimulation using the protocol for routine clinical laboratory cancer cytogenetics. After harvest, cells were treated with a hypotonic solution, fixed in methanol/acetic acid (3:1 ratio), and G banded using standard methods. Twenty cells in metaphase were routinely analyzed for karyotyping. To rule out the translocation involving IGH and CCND1 genes in CD5+ DLBCL, fluorescent in situ hybridization (FISH) study was performed on interphase nuclei using the LSI IGH/CCND1 dual-color, dual-fusion translocation probe (Vysis Inc., Downers Grove, IL, USA) based on the manufacturer's instructions.
Comparisons of clinical characteristics between the CD5+ and CD5 negative (CD5−) DLBCL were performed using a Chi-square test or Fisher's exact test for categorical variables. The overall survival (OS) was determined from the time of initial diagnosis to death from any cause or last follow-up. Progression-free survival (PFS) was determined from the time of initial diagnosis to disease progression, relapse, death, or last follow-up. The survival analyses were performed by Kaplan-Meier plots, and differences in survival were compared using the log-rank test. The 3-year OS and PFS of CD5+ and CD5− DLBCL were compared using the Z score. Univariate analysis was performed using Cox regression for variables including age, lactase dehydrogenase (LDH), B symptoms, IPI, ECOG performance status (PS), cell-of-origin, CD5 expression, and karyotype. P-values <0.05 were considered statistically significant. All statistical analyses were performed using the statistical Software Package for the Social Sciences (IBM SPSS Statistics 23; SPSS Inc., Chicago, IL, USA).
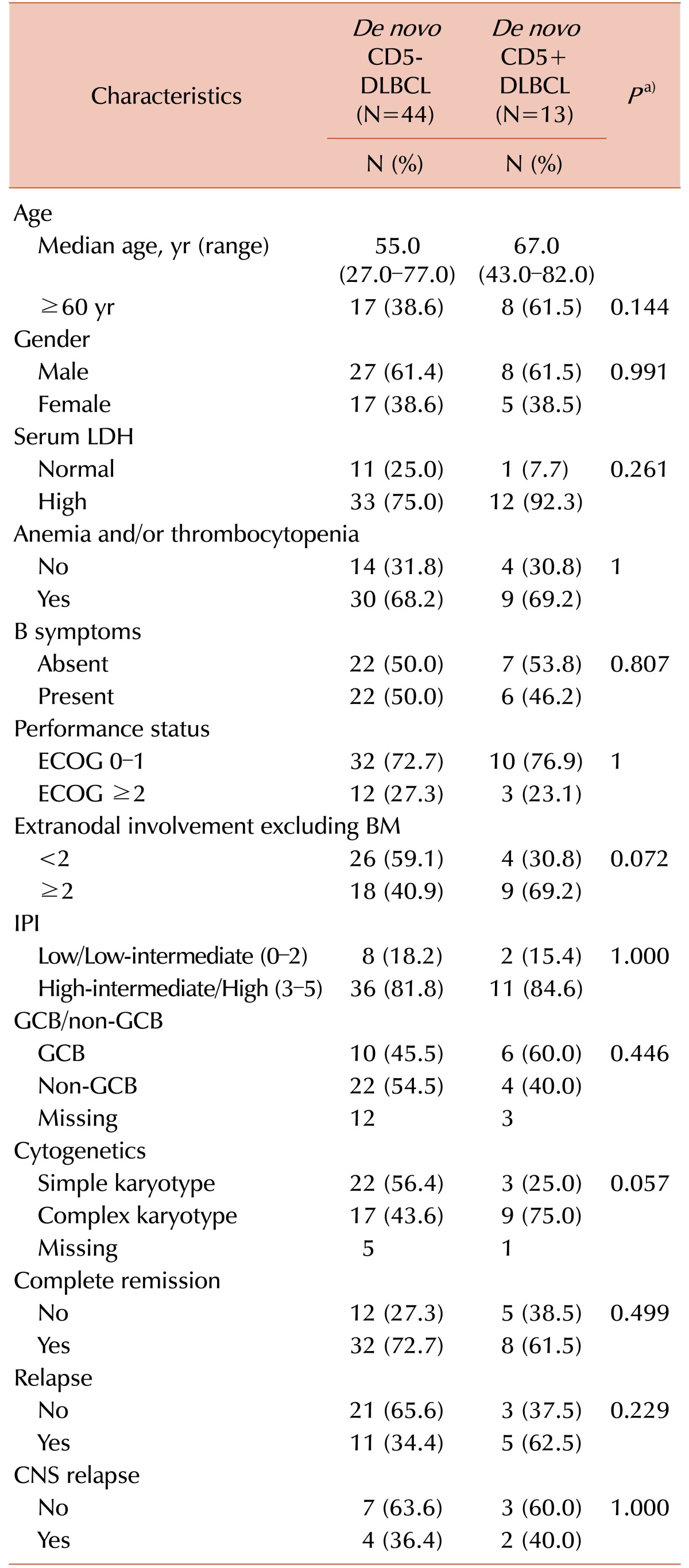
Among 768 patients with de novo DLBCL, BM involvement at diagnosis was observed in 76 patients (9.9%); among these, CD5 expression was evaluated in 57 patients. The number of patients with CD5+ and CD5− DLBCL were 13 and 44, respectively. In all patients with CD5+ DLBCL, except two patients who were not evaluated, no fusion signal was observed by the FISH study for IGH/CCND1. By Ann Arbor staging, all patients had stage IV disease. Median follow-up was 23 months (range, 0–88 mo). Clinical and laboratory characteristics of patients are summarized in Table 1. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) was the main chemotherapy regimen in both CD5+ and CD5− DLBCL groups. Fifty-four patients received chemotherapy with R-CHOP and one received Rituximab alone. Among the two patients who did not receive chemotherapy, one expired before chemotherapy and one was lost to follow-up. The median age of the patients was 56.0 years (range, 27.0–82.0 yr) and 56.1% were older than 60 years. LDH level was elevated in 78.9% of patients, 67.3% had anemia and/or thrombocytopenia, 49.1% had B symptoms, 26.3% had ≥2 of ECOG PS, 82.5% had ≥3 of IPI, 61.9% were non-GCB type; and 51.0% had complex karyotype (See Supplementary Table 1). In addition, 70.2% of patients achieved complete remission and among these, 40.0% relapsed after first remission. Clinical and laboratory characteristics of CD5+ DLBCL cases were not significantly different from those of CD5− DLBCL cases.
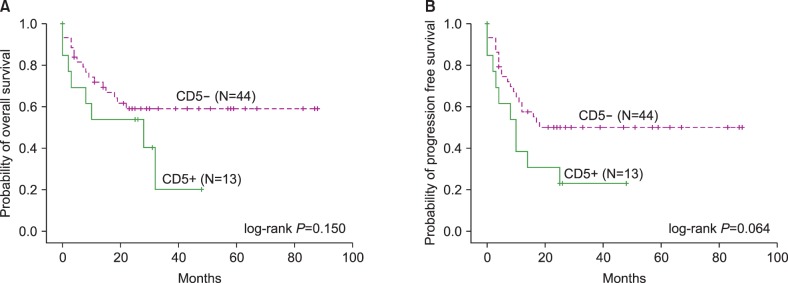
The median OS was not reached and the median PFS was 14 months in patients with DLBCL (Fig. 1). The 2-year OS and PFS were 58.0% and 45.6%, respectively. Survival analysis was also performed in subgroups based on CD5 expression, cell-of-origin, and IPI score. The median OS was 10 months in CD5+ DLBCL, and not reached in CD5− DLBCL. The 3-year OS rate was 20.2% and 59.0% in CD5+ and CD5− DLBCL, respectively (P=0.031). The 3-year PFS rate was 23.1% and 50.1% in CD5+ and CD5− DLBCL, respectively (P=0.055). However, the overall inferior trend of OS and PFS in CD5+ DLBCL was not statistically significant (OS, log-rank P=0.150; PFS, log-rank P=0.067) (Fig. 2). The OS and PFS were not different between patients with GCB and non-GCB DLBCL (OS, log-rank P=0.483; PFS, log-rank P=0.562). The median OS and PFS were 19 months and 10 months in patients with ≥3 IPI, and not reached in patients with <3 IPI. Patients with higher IPI exhibited significantly shorter OS and PFS (OS, log-rank P<0.001; PFS, log-rank P<0.001).
In univariate analysis, ECOG PS ≥2 (HR=4.344, 95% CI=1.847–10.218, P=0.001) and elevated LDH (HR=9.340, 95% CI=1.261–69.197, P=0.029) were associated with decreased OS in patients with DLBCL, and other factors did not provide significant prognostic impact.
In this study, we compared the clinicopathologic characteristics and prognosis of patients with BM involvement of CD5+ and CD5− DLBCL. Previously, Yamaguchi et al. [512], demonstrated a higher age distribution, female predominance, higher frequency of ≥2 of performance status, high LDH level, stage III or IV disease at diagnosis, and presence of B symptoms, high incidence of central nervous system recurrence and non-GCB type, and inferior overall survival in CD5+ DLBCL, as compared to CD5− DLBCL. In this study, although statistically significant differences were not observed, CD5+ DLBCL showed more aggressive features correlated with age, LDH level, and karyotype than CD5− DLBCL; patients with CD5+ DLBCL tended to be older than 60 years (CD5+ vs. CD5−; 62% vs. 39%, P=0.144) and exhibit elevated LDH level (92% vs. 75%, P=0.261) and complex karyotype (75% vs. 44%, P=0.057). Other clinical and laboratory characteristics including gender, anemia/thrombocytopenia, B symptoms, performance status ≥2, IPI score ≥3, and CNS relapse were comparable in both groups, possibly due to the advanced stages of disease by the BM involvement itself.
Regarding chromosomal abnormalities, Yoshioka et al. [13], reported that abnormalities of 8p21, 11q13, and 3q27 were common in CD5+ DLBCL; and Niitsu et al. [7], reported that translocations involving 19p13 were the most common. In this study, 11q13 abnormalities were absent, and 8p21 abnormalities were observed in two patients (4.5%) with CD5− and one patient (7.7%) with CD5+ DLBCL. Translocations of 19p13 were observed in each of the two patients with CD5− (4.5%) and CD5+ (15.4%) DLBCL. However, the number of patients with each chromosomal abnormality was insufficient to evaluate the association with CD5 expression.
BM involvement of DLBCL is reported in 10–20% of patients with DLBCL [141516]. In this study, the proportion of BM involvement of DLBCL at diagnosis was 9.9%, which was similar to the previously reported frequency of 9.2% in Korea [2]. BM is the most frequently involved extranodal site in CD5+ DLBCL, with a higher frequency than CD5− DLBCL [59]. Several studies have reported the poor prognostic impact of BM involvement or CD5 expression in DLBCL. Kajiura et al. [17] evaluated 37 patients with DLBCL initially manifesting in the BM; the mean survival was 14.9 months and approximately 70% of patients died within 2 years. Sehn et al. [18] demonstrated that 49–69% of 3-year OS, and Chung et al. [14], demonstrated 34.5% of 5-year OS in cases with BM involvement of DLBCL. For CD5+ DLBCL, Ennishi et al. [6], reported that 45% of 2-year OS and Alinari et al. [10], reported 65% and 40% of 3-year OS and PFS, respectively. Xu-Monette et al. [9] showed 35.5% and 29.6% of 5-year OS and PFS, respectively, in patients with CD5+ DLBCL treated with R-CHOP. In our study, the 3-year OS was significantly inferior in CD5+ DLBCL than CD5− DLBCL (20.2% vs. 59.0%, P=0.031), and this survival rate of 20.2% in CD5+ DLBCL was quite low compared to the previous studies that evaluated either BM involvement of DLBCL or CD5+ DLBCL regardless of BM involvement. However, statistically significant differences in OS and PFS were not reached between patients with BM involvement of CD5+ DLBCL and those of CD5− DLBCL (OS, log-rank P=0.150; PFS, log-rank P=0.067), although CD5+ DLBCL showed an inferior tendency in OS and PFS than CD5− DLBCL. These might be due to small sample size, and if more patients are enrolled, these results might be statistically significant.
By gene expression profiling, Miyazaki et al. [19], showed that most CD5+ DLBCL are activated B-cell like (ABC) type. However, in our study, the proportion of non-GCB type did not differ between patients with CD5+ and CD5− DLBCL (P=0.446), which might be due to the limitation of immunohistochemistry-based assessment for GCB/non-GCB [6] and relatively high proportion of missing patients not evaluated for cell-of-origin. In addition, there were no significant differences in OS and PFS between non-GCB and GCB of DLBCL (OS, log-rank P=0.483; PFS, log-rank P=0.562), which suggested that BM involvement in DLBCL results in a poor outcome regardless of the cell-of-origin.
For the IPI score, Chung et al. [14] showed that patients with BM involvement of DLBCL had higher IPI scores, as compared to the patients without BM involvement. In our study, approximately 83% of patients had an IPI score ≥3 and the proportion of patients with higher IPI score did not differ between CD5− and CD5+ DLBCL (P=1.000). However, a poor prognostic impact of higher IPI score was observed in all patients with DLBCL, suggesting the prognostic impact of IPI score even in patients with BM involvement.
Our study had some limitations, including the small number of enrolled patients and the short follow-up duration. Since BM involvement of DLBCL was infrequent and not all patients with BM involvement were evaluated for CD5 expression, we could not include a sufficient number of patients. In addition, the patients with DLBCL without BM involvement were not included as a control group. However, we reviewed the previous studies evaluating CD5+ DLBCL regardless of BM involvement and compared the previously reported survival rates with those from our study.
We evaluated the prognostic significance of CD5 expression in patients with BM involvement of DLBCL. The results indicated that patients with CD5+ DLBCL showed an inferior survival tendency than patients with CD5− DLBCL. Thus, a thorough evaluation of CD5 expression might be helpful for prognosis prediction in patients with DLBCL. Further studies with a larger study population are needed to confirm the significance of CD5 expression in Korean patients with DLBCL.
References
1. Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC Press;2008.
2. Yi HG, Kim JS, Suh C, et al. Clinical features and survival outcomes of patients with diffuse large B-cell lymphoma: analysis of web-based data from the Korean Lymphoma Working Party Registry. Blood Res. 2013; 48:115–120. PMID: 23826580.

3. International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993; 329:987–994. PMID: 8141877.
4. Yamaguchi M, Ohno T, Oka K, et al. De novo CD5-positive diffuse large B-cell lymphoma: clinical characteristics and therapeutic outcome. Br J Haematol. 1999; 105:1133–1139. PMID: 10554834.

5. Yamaguchi M, Seto M, Okamoto M, et al. De novo CD5+ diffuse large B-cell lymphoma: a clinicopathologic study of 109 patients. Blood. 2002; 99:815–821. PMID: 11806981.

6. Ennishi D, Takeuchi K, Yokoyama M, et al. CD5 expression is potentially predictive of poor outcome among biomarkers in patients with diffuse large B-cell lymphoma receiving rituximab plus CHOP therapy. Ann Oncol. 2008; 19:1921–1926. PMID: 18573805.

7. Niitsu N, Okamoto M, Tamaru JI, et al. Clinicopathologic characteristics and treatment outcome of the addition of rituximab to chemotherapy for CD5-positive in comparison with CD5-negative diffuse large B-cell lymphoma. Ann Oncol. 2010; 21:2069–2074. PMID: 20231297.

8. Miyazaki K, Yamaguchi M, Suzuki R, et al. CD5-positive diffuse large B-cell lymphoma: a retrospective study in 337 patients treated by chemotherapy with or without rituximab. Ann Oncol. 2011; 22:1601–1607. PMID: 21199885.

9. Xu-Monette ZY, Tu M, Jabbar KJ, et al. Clinical and biological significance of de novo CD5+ diffuse large B-cell lymphoma in Western countries. Oncotarget. 2015; 6:5615–5633. PMID: 25760242.

10. Alinari L, Gru A, Quinion C, et al. De novo CD5+ diffuse large B-cell lymphoma: Adverse outcomes with and without stem cell transplantation in a large, multicenter, rituximab treated cohort. Am J Hematol. 2016; 91:395–399. PMID: 26800311.

11. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004; 103:275–282. PMID: 14504078.

12. Yamaguchi M, Nakamura N, Suzuki R, et al. De novo CD5+ diffuse large B-cell lymphoma: results of a detailed clinicopathological review in 120 patients. Haematologica. 2008; 93:1195–1202. PMID: 18556402.

13. Yoshioka T, Miura I, Kume M, et al. Cytogenetic features of de novo CD5-positive diffuse large B-cell lymphoma: chromosome aberrations affecting 8p21 and 11q13 constitute major subgroups with different overall survival. Genes Chromosomes Cancer. 2005; 42:149–157. PMID: 15543600.

14. Chung R, Lai R, Wei P, et al. Concordant but not discordant bone marrow involvement in diffuse large B-cell lymphoma predicts a poor clinical outcome independent of the International Prognostic Index. Blood. 2007; 110:1278–1282. PMID: 17475910.

15. Chigrinova E, Mian M, Scandurra M, et al. Diffuse large B-cell lymphoma with concordant bone marrow involvement has peculiar genomic profile and poor clinical outcome. Hematol Oncol. 2011; 29:38–41. PMID: 20635329.

16. Park MJ, Park SH, Park PW, et al. Prognostic impact of concordant and discordant bone marrow involvement and cell-of-origin in Korean patients with diffuse large B-cell lymphoma treated with R-CHOP. J Clin Pathol. 2015; 68:733–738. PMID: 25998512.

17. Kajiura D, Yamashita Y, Mori N. Diffuse large B-cell lymphoma initially manifesting in the bone marrow. Am J Clin Pathol. 2007; 127:762–769. PMID: 17439835.

18. Sehn LH, Scott DW, Chhanabhai M, et al. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol. 2011; 29:1452–1457. PMID: 21383296.

19. Miyazaki K, Yamaguchi M, Imai H, et al. Gene expression profiling of diffuse large B-Cell lymphomas supervised by CD5 expression. Int J Hematol. 2015; 102:188–194. PMID: 26009281.

SUPPLEMENTARY MATERIAL
Supplementary Table 1
Cytogenetic study results of 57 patients with bone marrow involvement of de novo diffuse large B cell lymphoma (DLBCL).
Fig. 1
Kaplan-Meier plots for the overall survival (OS) and progression-free survival (PFS) in de novo diffuse large B cell lymphoma. Median OS was not reached, and median PFS was 14 months.
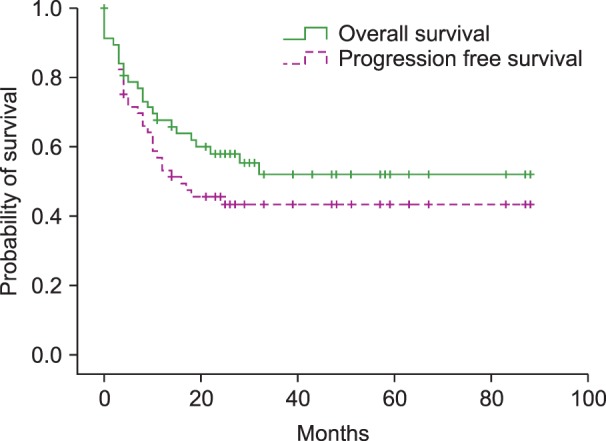
Fig. 2
Kaplan-Meier plots for the (A) overall survival and (B) progression-free survival in de novo diffuse large B cell lymphoma according to CD5 expression.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download