Abstract
Background
Standard remission induction chemotherapy consisting of anthracycline plus cytarabine (3+7) is administered for adult acute myeloid leukemia (AML). However, the effects of intensified regimen on complete remission (CR), relapse and overall survival (OS) remain unknown.
Methods
We analyzed 1195 patients treated with idarubicin plus cytarabine/BHAC (3+7) from 2002 to 2013. Among them, 731 received early intensification with 3-day cytarabine/BHAC (3+10, N=363) or 2-day idarubicin plus cytarabine/BHAC 3 days (5+10, N=368). The 3+10 and 5+10 strategies were applied to patients with bone marrow blast counts of 5–20% and >20% on day 7 of 3+7, respectively.
Results
Early intensification correlated with a younger age (median: 40 vs. 45 yr) and higher t(8;21) frequency (20.4% vs. 7.1%), compared to 3+7. After early intensification, the early death rates were higher among the elderly (3+10 [15.7%], 5+10 [21.7%] vs. 3+7 [6.3%], P=0.038), while the post-induction CR rate was higher in young patients (3+10 [79.8%], 5+10 [75.1%] vs. 3+7 [65.1%], P<0.001). Early relapse rate was also decreased (3+10 [11.8%], 5+10 [11.7%] vs. 3+7 [22.0%], P<0.001). In multivariate analysis, early intensification correlated with an inferior 5-year OS among elderly patients (19.2% vs. 22.8%; hazard ratio [HR]=1.84, 95% confidence interval [CI]; 1.11–3.06, P=0.018) and lower overall relapse rate among young patients (33.0% vs. 41.4%, P=0.023; HR=0.71, 95% CI; 0.55–0.93, P=0.012).
The current standard remission induction chemotherapy strategy for adult patients younger than 60 years with acute myeloid leukemia (AML) consisting of a continuous standard doses of cytarabine infusion (100–200 mg/m2) for 7 days combined with either idarubicin (12 mg/m2 for 3 days) or daunorubicin (40–60 mg/m2 for 3 days) [12], has not changed considerably over the last 25 years. However, several previous trials have used novel agents or dose-escalation methods to intensify this induction regimen for improving the hematological complete remission (CR) rate and reducing the relapse rate, which would yield better survival outcomes.
Initially, different types or doses of anthracyclines were compared to evaluate possible improvements in clinical outcomes. A recent meta-analysis of 29 randomized controlled trials comparing the efficacies of idarubicin and daunorubicin at different dosing schedules revealed that idarubicin reduced the remission failure rate, as indicated by a risk ratio of 0.81 (95% confidence interval [CI]; 0.66–0.99, P=0.04) only when the daunorubicin/idarubicin dose ratio was <5, and did not significantly affect the outcomes of early death or overall mortality [3]. Few studies have reported a reduced remission failure rate with escalated doses of daunorubicin (60–90 mg/m2), compared to the standard dose (45 mg/m2) [456] or idarubicin [7]. Recent data also demonstrated the benefit of high-dose daunorubicin regardless of the cytogenetic risk, even in patients with the FLT3-ITD and DNMT3A mutations [8]. Other studies have also evaluated high-dose cytarabine for induction therapy and revealed that intensified regimen increases the treatment-related mortality and toxicity rates in spite of benefits for some patient subgroups [91011]. One prospective trial suggested that high-dose cytarabine yielded superior overall survival (OS) without excessive toxicity in younger patients and high-risk patients with high-risk cytogenetic profiles and/or the FLT3-ITD mutation or those with secondary AML, whereas no benefits were observed for elderly patients [12]. A recent meta-analysis also showed that high-dose cytarabine improved survival outcomes and reduced relapses, particularly among AML patients with favorable-risk cytogenetic profiles; however, toxicity was a limiting factor [13].
The adult AML patients visiting our healthcare center were initially treated with a protocol comprising 3 days of idarubicin and 7 days of intermediate-dose cytarabine or N4-behenoyl-1-β-D-arabinofuranosyl cytosine (BHAC). And then, they were administered early intensified induction therapy according to the bone marrow (BM) blast counts on day 7 of the initial chemotherapy regimen. We tried to identify the treatment outcomes associated with early intensification compared to those of the standard 3+7 regimen in Korean AML patients, with the aim of demonstrating the effect of additional cytarabine or dose-escalated idarubicin therapy.
After excluding patients with acute promyelocytic leukemia, we retrospectively enrolled 1,195 adult AML patients who were initially treated with idarubicin-based intensive induction chemotherapy between 2002 and 2013. The diagnoses were established by morphological, cytochemical, immunophenotypic, and cytogenetic analyses of BM blast cells. For karyotyping, at least 20 metaphase cells were analyzed using the GTG banding method and the International System for Cytogenetic Nomenclature (ISCN) [14]. For the molecular analysis, we screened 28 genetic aberrations by multiplex reverse transcriptase polymerase chain reaction (RT-PCR) using the HemaVision Kit (DNA Technology, Aarhus, Denmark). Unfortunately, molecular studies, including the detection of NPM1, CEBPα, FLT3, and c-kit mutations and of WT1 and BAALC expression, were not generally implemented before 2008. As a result, cases without molecular marker data were stratified according to the karyotype alone. For the final molecular cytogenetic risk-stratification, we divided patients into three subgroups according to the National Comprehensive Cancer Network (NCCN) guidelines [2]. This research was conducted in accordance with the Institutional Review Board and Ethics Committee guidelines of the Catholic Medical Center (approval number: KC17RESI0156) and the principles of the Declaration of Helsinki.
Fig. 1 shows the treatment strategy for adult AML used at the Catholic Blood and Marrow Transplantation Center in Korea. All patients were initially treated with 3+7 induction chemotherapy, comprising idarubicin (12 mg/m2) for 3 days plus cytarabine (100 mg/m2) or BHAC (300 mg/m2) for 7 days [15]. Of these patients, 731 (61.2%) additionally received continuous early intensification with cytarabine or BHAC for the following 3 days (3+10, N=363) or idarubicin for the following 2 days and cytarabine or BHAC for the following 3 days (5+10, N=368). Decisions for early intensification were based on the follow-up BM blast counts on day 7 of 3+7 induction chemotherapy; 3+10 intensification was administered for blast counts of 5–20% and 5+10 intensification was administered for blast counts >20% (early intensification group). The remaining 464 patients with blast counts <5% finished the 3+7 regimen without early intensification (standard group). For patients who achieved a hematological CR after induction, we applied our standard consolidation chemotherapy, which consisted of a 3+5 regimen comprising mitoxantrone (12 mg/m2) or idarubicin (12 mg/m2) for 3 days plus an intermediate dose of cytarabine (1.0 g/m2 every 12 hr) or BHAC (300 mg/m2) for 5 days; these were alternatively applied. For patients who did not achieve remission, we administered re-induction chemotherapy, which comprised 4 days of mitoxantrone (10 mg/m2) and intermediate-dose cytarabine (1.0 g/m2 every 12 hr), followed by 3 days of etoposide (100 mg/m2).
For patients in CR, we searched for available donors for allogeneic-hematopoietic cell transplantation (HCT) during the consolidation period, giving initial preference to human leukocyte antigen (HLA)-matched sibling donors (MSDs), followed by HLA well-matched unrelated donors (URDs). When conventional donors were not available, we searched for haploidentical familial mismatched donors; if a patient refused allogeneic-HCT, we performed autologous-HCT or completed three cycles of consolidation chemotherapy alone according to the patient's and physician's joint decision [161718]. If a patient was a candidate for autologous-HCT, CD34+ hematopoietic stem cells were collected for 3 days during consolidation chemotherapy after the neutrophil count had recovered. For donor mobilization, we administered granulocyte-colony stimulating factor subcutaneously at a dose of 10 µg/kg/day for 4 days. Patients who underwent HCT received either a myeloablative (MAC) or a reduced-intensity (RIC) conditioning regimen. Briefly, the MAC regimen comprised cyclophosphamide (120 mg/kg) combined with total body irradiation (TBI; 1320 cGy) or busulfan (12.8 mg/kg). The autologous MAC regimen comprised TBI (1200 cGy) plus ARA-C (9 g/body surface area) and melphalan (100 mg/body surface area) [19]. For the RIC regimen, we administered busulfan (6.4 mg/kg) and fludarabine (150 mg/m2) with TBI (400 cGy) [20]. Anti-thymocyte globulin (ATG) at a dose of 2.5 mg/kg (1.25 mg/kg each on days -3 and -2) was administered to patients receiving stem cells from an URD. For haploidentical transplantation, we administered fludarabine (150 mg/m2) and busulfan (6.4 mg/kg) with TBI (800 cGy) and ATG (5 mg/kg in 1.25 mg/kg doses on days -4 to -1) [21].
This study was conducted to assess clinical outcomes according to induction chemotherapy intensity, and focused on early death (within 8 weeks), early relapse during or after the consolidation period before HCT, long-term overall survival (OS) and the cumulative incidence of overall relapse (CIR). All categorical variables were compared using a chi-squared analysis and Fisher's exact test, and continuous variables were assessed using Student's t-test and the Wilcoxon rank-sum test. OS was estimated using a Kaplan-Meier analysis, and a log-rank analysis was used to evaluate differences in survival between the groups. The cumulative incidence of early death or early relapse and the CIR were estimated using a cumulative incidence estimation method that treated relapse and non-relapse deaths as competing risks, and were compared using the Gray test [22]. A multivariate analysis based on the Cox proportional regression model was used to calculate survival hazard ratios, and the Fine–Gray proportional hazard regression model was used to calculate the hazard ratios for cumulative incidences. All statistical analyses were performed using “R” software (version 2.15.1; R Foundation for Statistical Computing, Vienna, Austria, 2012). Statistical significance was set at a P-value <0.05.
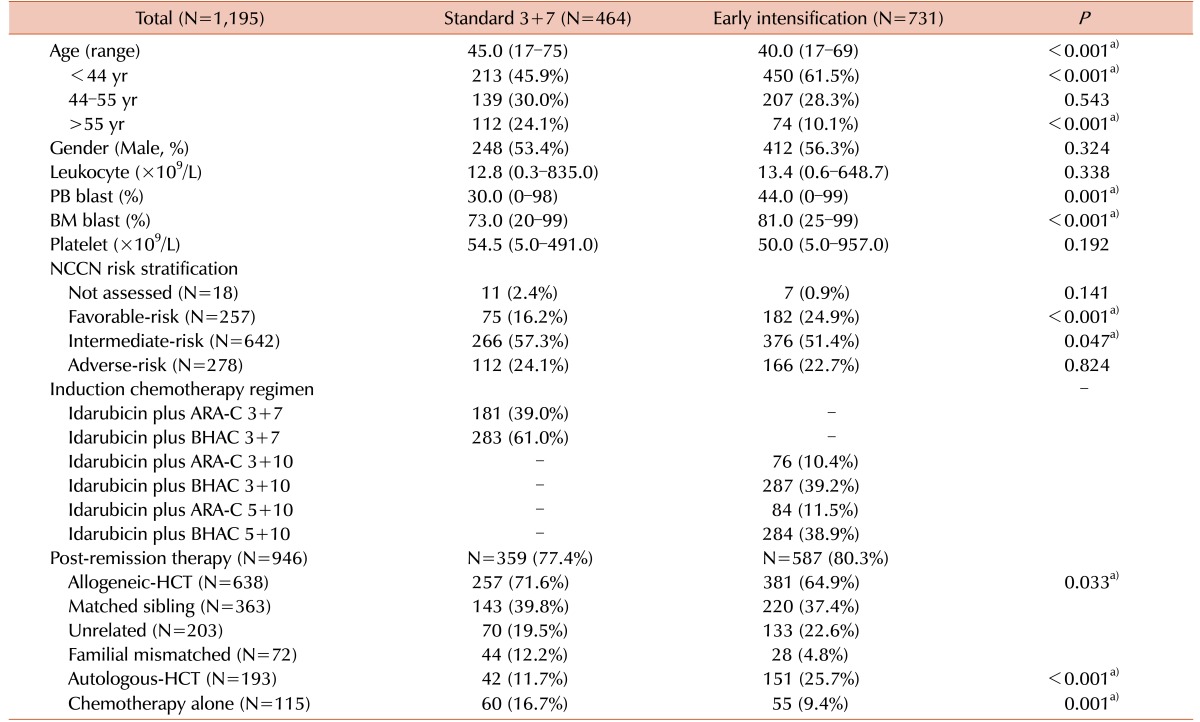
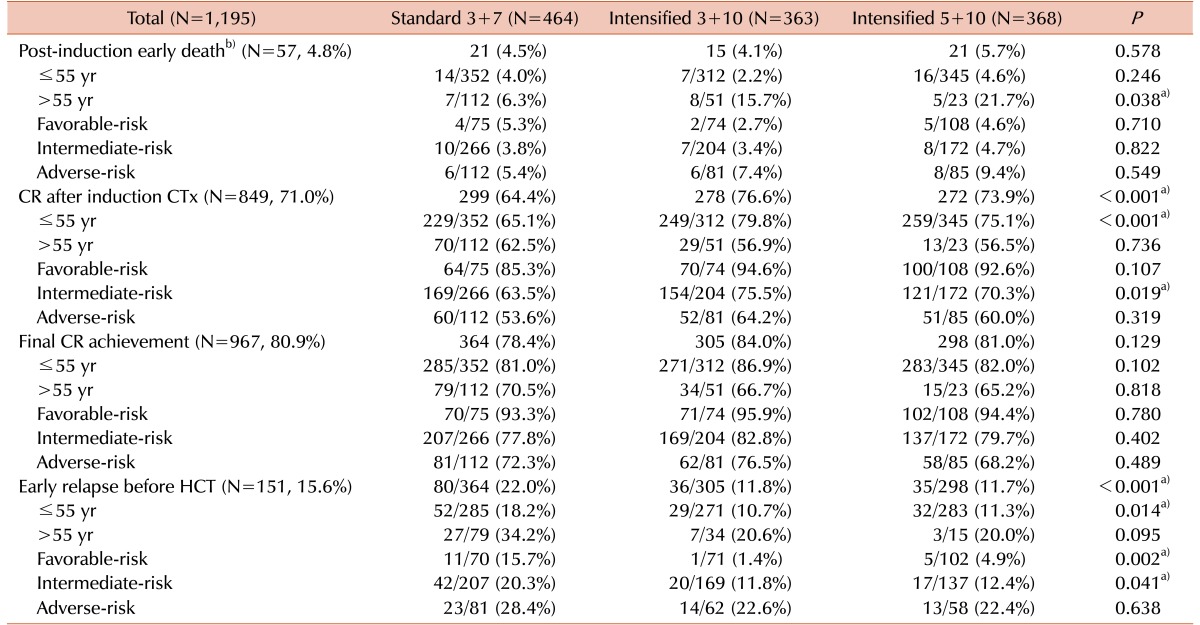
The baseline characteristics of the two subgroups according to the induction chemotherapy induction are shown in Table 1. The standard group (N=464) and the early intensification group (N=731) had median ages of 45 years (range, 17–75 yr) and 40 years (range, 17–69 yr), respectively (P<0.001). The early intensification group had a higher proportion of young patients (≤55 yr) (89.9% vs. standard: 75.9%, P<0.001). Furthermore, the early intensification group had a significantly higher peripheral blood blast count (44% vs. 30%, P=0.001), BM blast count (81% vs. 73%, P<0.001), and frequency of t(8;21) (20.4% vs. 7.1%, P<0.001). Among the 182 patients harboring t(8;21), 56.0% (N=102) had a BM blast count >20% and 25.8% (N=47) had a BM blast count of 5–20% on day 7 of induction chemotherapy and accordingly received early intensification therapy. In our cohort, overall 79.1% (N=946) of patients, consisting of 77.4% (N=359) of standard group and 80.3% (N=587) of early intensification group, received postremission therapy after achieving a CR (P=0.224). Significantly higher percentage of patients of standard group received allogeneic-HCT (71.6% vs. 64.9%, P=0.033) and repeated consolidation chemotherapy (16.7% vs. 9.4%, P=0.001).
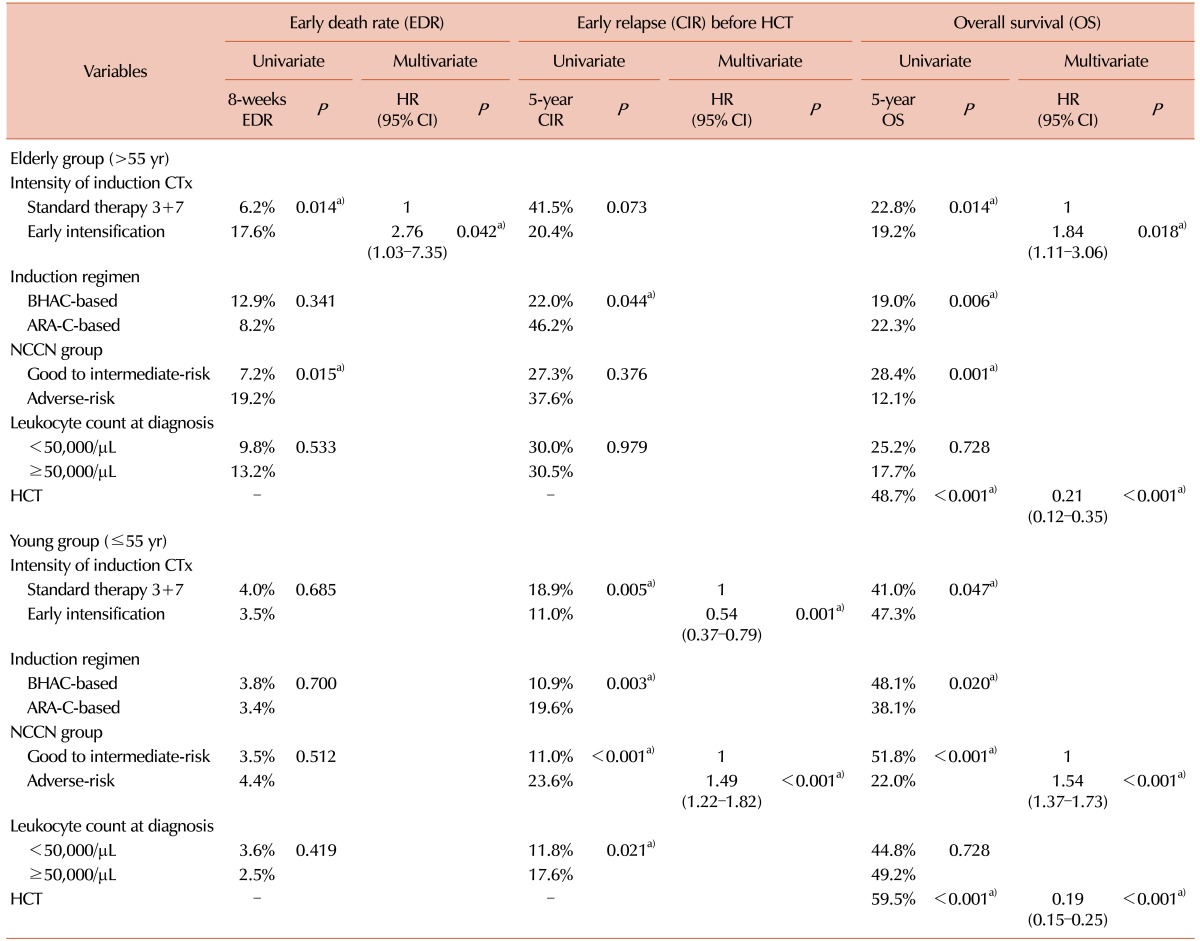
We calculated the early death rate (up to 8 weeks after induction therapy), CR rate and early relapse rate before HCT according to the induction chemotherapy intensity (Table 2). The early death rate was 4.8% (N=47) and significantly higher in early intensification group, especially among patients older than 55 years (17.5% vs. 6.3%, P=0.015). A total of 849 (71.0%) patients achieved a CR after one cycle of induction chemotherapy, and 967 (80.9%) achieved a CR after re-induction chemotherapy. Although the final CR rates did not differ significantly even after early intensification, the CR rate after one cycle of induction chemotherapy was significantly higher in early intensification group (76.6%, 73.9% and 64.4% in the 3+10 group, 5+10 group and standard group, respectively, P<0.001) especially among patients younger than 55 years (79.8%, 75.1% and 65.1% in the 3+10 group, 5+10 group and standard group, respectively, P<0.001) and those in the intermediate-risk group (75.5%, 70.3% and 63.5% in the 3+10 group, 5+10 group and standard group, respectively, P=0.019). The overall early relapse rate before HCT was 15.6%, and significantly decreased in early intensification group (11.8%, 11.7% and 22.0% in the 3+10 group, 5+10 group and standard group, respectively, P<0.001) except for adverse-risk group. In the favorable-risk group, the early relapse rate was significantly lower in early intensification group (1.4%, 4.9% and 15.7% in the 3+10 group, 5+10 group and standard group, respectively, P=0.002). The early clinical outcomes were not significantly different between the two intensification groups. Although the cumulative incidence of early death did not significantly differ between the standard and early intensification groups (4.5% vs. 4.9%, P=0.768, Fig. 2A), the cumulative incidence of early relapse before HCT was significantly lower in early intensification group (11.8% vs. 20.9%, P<0.001, Fig. 2B). In the elderly group (>55 yr), however, the cumulative incidence of early death was significantly higher in the early intensification group (17.6% vs. 6.2%, P=0.014, Fig. 2C). In the young patient group (≤55 yr), although the early death rate did not significantly differ with respect to treatment intensity (3.5% vs. 4.0%, P=0.685), the cumulative incidence of early relapse before HCT was significantly lower after early intensification (11.0% vs. 18.9%, P=0.005, Fig. 2D). As shown in Table 3, the multivariate analysis identified that early intensification significantly increased early death rate in the elderly group (HR=2.76, 95% CI: 1.03–7.35, P=0.042) and significantly reduced early relapse rate before HCT only in the young patient group (HR=0.54, 95% CI: 0.37–0.79, P=0.001).
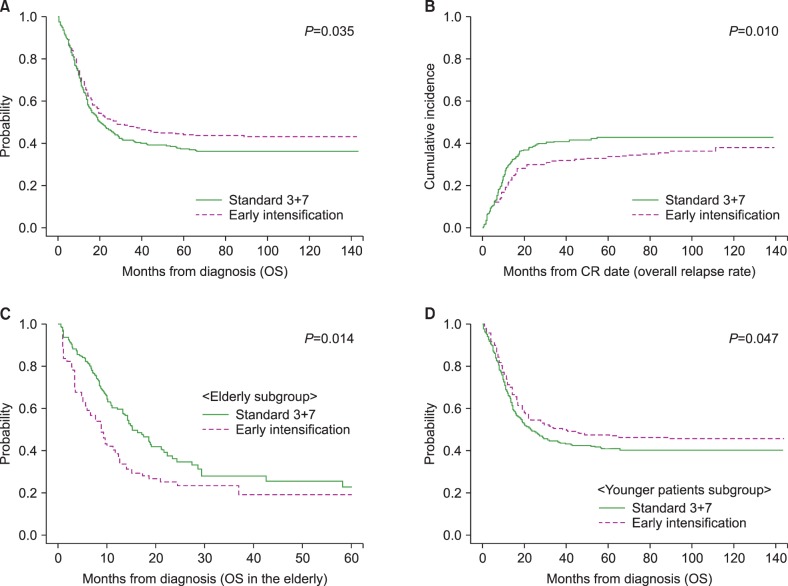
Overall, early intensification was associated with a superior 5-year OS (44.4% vs. 37.4%, P=0.035, Fig. 3A) and lower relapse rate (33.6% vs. 42.7%, P=0.010, Fig. 3B), compared with standard treatment during a median follow-up of 54.5 months (range, 5.8–143.8). In the elderly group, early intensification correlated with early death and, consequently, an inferior 5-year OS (19.2% vs. 22.8%, P=0.014, Fig. 3C), as confirmed by the multivariate analysis (HR=1.84, 95% CI: 1.11–3.06, P=0.018). In contrast, early intensification correlated with a reduced rate of early relapse and, consequently, a superior 5-year OS (47.3% vs. 41.0%, P=0.047, Fig. 3D) and reduced 5-year overall relapse rate (33.0% vs. 41.4%, P=0.023) in the young patient group. In multivariate analysis, only the factors including early intensification (HR=0.71, 95% CI: 0.55–0.93, P=0.012), intermediate-to-favorable molecular cytogenetics (HR=0.38, 95% CI: 0.29–0.48, P<0.001) and HCT (HR=0.25, 95% CI: 0.17–0.37, P<0.001) were associated with a lower overall relapse rate, while no significant effect of early intensification on long-term OS was confirmed (Table 3).
Our current study demonstrated that early intensification with additional cytarabine or BHAC for 3 days, with or without an additional 2 days of idarubicin therapy, was associated with a higher early death rate and inferior OS in elderly group, despite higher post-induction CR rate and lower early relapse rate before HCT. However, both univariate and multivariate analysis showed that early intensification was associated with lower overall relapse rate in young patient group. In addition, early-intensification had no effect on clinical outcome in adverse-risk group. Therefore, we suggest that early intensification may benefit young patients without an adverse-risk molecular cytogenetic profile. Among patients in the adverse-risk category, clinical trials should be first consider the use of several novel FLT3-ITD or c-kit mutation-targeting agents before proceeding to allogeneic-HCT.
We mainly selected BHAC, widely used in Japan due to lower incidence of toxicities such as nausea and vomiting, as initial chemotherapy regimen [23]. Owing to its lower CR rate and poorer survival outcome compared to cytarabine [24], we attempted to intensify the induction regimen by adding 3 further days of BHAC with or without 2 days of idarubicin therapy, according to the BM blast count on the final chemotherapy infusion day during the initial 3+7 regimen. However, we mainly replaced BHAC with cytarabine within the same intensification strategy since 2009. We found that our current results were mainly affected by the outcomes of treatment with the intensification strategy comprising BHAC plus idarubicin, which was associated with a relatively lower early death rate (12.2% in 3+10 and 18.8% in 5+10) even in the elderly group (Supplementary Table 1). In comparison, cytarabine-based intensification did not increase the post-induction CR rate, but was associated with higher early death rates (30.0% in 3+10 and 28.6% in 5+10) in the elderly group and lower early relapse rates in the favorable-risk group (Supplementary Table 2). Therefore, BHAC-based intensification might be more appropriate than cytarabine-based intensification, which yielded increased rates of toxicity and non-relapse mortality in the elderly patients.
Our data showed that AML patients with the t(8;21) mutation were more likely to receive early intensification as a result of high remnant BM blast counts on day 7 of the 3+7 chemotherapy regimen, suggesting their late blast clearance, although t(8;21) is considered a favorable-risk cytogenetic marker. However, all patients with favorable-risk cytogenetics had an optimal response (blast ≤5%) and completed induction therapy without double induction on day 14 of induction chemotherapy. Several previous reports showed correlations of an early BM blast clearance with a higher CR rate and better survival outcomes [252627], and many guidelines suggest that a second induction is recommended for patients with high remnant BM blasts on day 14 of induction chemotherapy. Therefore, our BM blast evaluation on day 7 was too early to predict the clinical outcomes for some patients such as those with the t(8;21) mutation. Nevertheless, our current study revealed that the administration of early intensification based on this earlier BM blast evaluation might be an acceptable remission induction strategy for young patients with intermediate-to-favorable-risk molecular cytogenetics, particularly with regard to a higher CR rate and lower early relapse rate. Given our experiences with a very long neutropenic period and higher mortality associated with additional 3+7 chemotherapy according to the BM blast count on day 14, it seems that the well-known Western protocol might be very toxic for Asian (and specifically Korean) patients. Therefore, based on our current results, we are currently planning a well-designed prospective trial to prove the role of BM blast evaluation on day 7 and the usefulness of early intensification for selected groups of AML patients.
After the consolidation chemotherapy comprising intermediate-dose cytarabine or BHAC combined with idarubicin, the patients underwent allogeneic-HCT when a well matched donor was available. Otherwise, the patients underwent autologous-HCT followed by chemotherapy alone or haploidentical HCT. Although high-dose cytarabine was recommended a standard consolidation regimen for patients younger than 60 years with intermediate-to-favorable-risk cytogenetics [28], we used an intermediate dose of cytarabine due to the risks of neurologic toxicity and non-relapse mortality associated with high-dose cytarabine and previous reports that demonstrated similar treatment outcomes with intermediate-dose cytarabine [2930].
Considering the effects of consolidation therapy on survival and many confounding parameters in HCT, it is difficult to properly evaluate the long-term survival outcomes of each induction regimens. However, we expect that the high post-induction CR rate and lower early death rate of early intensification in the young patient group might increase the likelihood for undergoing HCT leading to better disease-free survival. Unfortunately, however, early intensification did not significantly improve survival outcomes, although it significantly reduced the early relapse rate before HCT without increasing the early death rate.
Although this study was a retrospective analysis of data collected from a heterogeneous cohort over a long period, our observations were based on a consistent (i.e., largely unchanging over time) treatment strategy including consolidation chemotherapy, donor searching, pre-HCT conditioning regimens, immunosuppressive agents and supportive management. In conclusion, we suggest that early intensification may benefit young patients, especially those who have been treated with a BHAC-based regimen, and could result in a higher CR rate and lower relapse rate. However, this early intensification should not be applied to the elderly patients treated with a cytarabine-based regimen or patients with adverse-risk molecular cytogenetics.
Notes
References
1. Löwenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999; 341:1051–1062. PMID: 10502596.

2. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Acute Myeloid Leukemia (Version 1.2016). Fort Washington, PA: National Comprehensive Cancer Network;2016. Accessed March 5, 2017. https://www.nccn.org/professionals/physician_gls/PDF/aml.pdf.
3. Teuffel O, Leibundgut K, Lehrnbecher T, Alonzo TA, Beyene J, Sung L. Anthracyclines during induction therapy in acute myeloid leukaemia: a systematic review and meta-analysis. Br J Haematol. 2013; 161:192–203. PMID: 23398482.

4. Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009; 361:1249–1259. PMID: 19776406.

5. Lee JH, Joo YD, Kim H, et al. A randomized trial comparing standard versus high-dose daunorubicin induction in patients with acute myeloid leukemia. Blood. 2011; 118:3832–3841. PMID: 21828126.

6. Burnett AK, Russell NH, Hills RK, et al. A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood. 2015; 125:3878–3885. PMID: 25833957.
7. Pautas C, Merabet F, Thomas X, et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J Clin Oncol. 2010; 28:808–814. PMID: 20048183.

8. Luskin MR, Lee JW, Fernandez HF, et al. Benefit of high-dose daunorubicin in AML induction extends across cytogenetic and molecular groups. Blood. 2016; 127:1551–1558. PMID: 26755712.

9. Weick JK, Kopecky KJ, Appelbaum FR, et al. A randomized investigation of high-dose versus standard-dose cytosine arabinoside with daunorubicin in patients with previously untreated acute myeloid leukemia: a Southwest Oncology Group study. Blood. 1996; 88:2841–2851. PMID: 8874180.

10. Bishop JF, Matthews JP, Young GA, et al. A randomized study of high-dose cytarabine in induction in acute myeloid leukemia. Blood. 1996; 87:1710–1717. PMID: 8634416.
11. Bishop JF, Matthews JP, Young GA, Bradstock K, Lowenthal RM. Intensified induction chemotherapy with high dose cytarabine and etoposide for acute myeloid leukemia: a review and updated results of the Australian Leukemia Study Group. Leuk Lymphoma. 1998; 28:315–327. PMID: 9517503.

12. Willemze R, Suciu S, Meloni G, et al. High-dose cytarabine in induction treatment improves the outcome of adult patients younger than age 46 years with acute myeloid leukemia: results of the EORTC-GIMEMA AML-12 trial. J Clin Oncol. 2014; 32:219–228. PMID: 24297940.

13. Li W, Gong X, Sun M, et al. High-dose cytarabine in acute myeloid leukemia treatment: a systematic review and meta-analysis. PLoS One. 2014; 9:e110153. PMID: 25299623.

14. Shaffer LG, Slovak ML, Campbell LJ, editors. ISCN 2009: An International System for Human Cytogenetic Nomenclature. 2009. Basel, Switzerland: Karger.
15. Park HS, Kim DW, Kim CC, et al. Induction chemotherapy with idarubicin plus N4-behenoyl-1-beta-D-arabinofuranosylcytosine in acute myelogenous leukemia: a newly designed induction regimen--a prospective, cooperative multicenter study. Semin Hematol. 1996; 33(4 Suppl 3):24–29.
16. Yoon JH, Kim HJ, Park SS, et al. Long-term clinical outcomes of hematopoietic cell transplantation for intermediate-to-poor-risk acute myeloid leukemia during first remission according to available donor types. Oncotarget. 2017; 8:41590–41604. PMID: 28206975.

17. Yoon JH, Kim HJ, Park SS, et al. Clinical outcome of autologous hematopoietic cell transplantation in adult patients with acute myeloid leukemia: Who may benefit from autologous hematopoietic cell transplantation? Biol Blood Marrow Transplant. 2017; 23:588–597. PMID: 28089879.

18. Cho BS, Yoon JH, Shin SH, et al. Comparison of allogeneic stem cell transplantation from familial-mismatched/haploidentical donors and from unrelated donors in adults with high-risk acute myelogenous leukemia. Biol Blood Marrow Transplant. 2012; 18:1552–1563. PMID: 22516055.

19. Kim HJ, Min WS, Eom KS, et al. Autologous stem cell transplantation using modified TAM or combination of triple-alkylating agents conditioning regimens as one of the post-remission treatments in patients with adult acute myeloid leukemia in first complete remission. Bone Marrow Transplant. 2004; 34:215–220. PMID: 15170169.

20. Lee SE, Kim HJ, Min WS, et al. Favorable outcomes of intravenous busulfan, fludarabine, and 400 cGy total body irradiation-based reduced-intensity conditioning allogeneic stem cell transplantation for acute myelogenous leukemia with old age and/or co-morbidities. Int J Hematol. 2010; 92:342–350. PMID: 20694843.
21. Yahng SA, Kim JH, Jeon YW, et al. A well-tolerated regimen of 800 cGy TBI-fludarabine-busulfan-ATG for reliable engraftment after unmanipulated haploidentical peripheral blood stem cell transplantation in adult patients with acute myeloid leukemia. Biol Blood Marrow Transplant. 2015; 21:119–129. PMID: 25300871.

22. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988; 16:1141–1154.

23. Kimura K, Ohno R, Amaki I, et al. Phase I clinical and pharmacokinetic study of N4-behenoyl-1-beta-D-arabinofuranosylcytosine. Med Oncol Tumor Pharmacother. 1986; 3:15–24. PMID: 3702507.

24. Kobayashi T, Miyawaki S, Tanimoto M, et al. Randomized trials between behenoyl cytarabine and cytarabine in combination induction and consolidation therapy, and with or without ubenimex after maintenance/intensification therapy in adult acute myeloid leukemia. The Japan Leukemia Study Group. J Clin Oncol. 1996; 14:204–213. PMID: 8558199.

25. Campuzano-Zuluaga G, Deutsch Y, Salzberg M, et al. Routine interim disease assessment in patients undergoing induction chemotherapy for acute myeloid leukemia: Can we do better? Am J Hematol. 2016; 91:277–282. PMID: 26663264.

26. Haferlach T, Kern W, Schoch C, et al. A new prognostic score for patients with acute myeloid leukemia based on cytogenetics and early blast clearance in trials of the German AML Cooperative Group. Haematologica. 2004; 89:408–418. PMID: 15075074.
27. Kern W, Haferlach T, Schoch C, et al. Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: data from the German AML Cooperative Group (AMLCG) 1992 Trial. Blood. 2003; 101:64–70. PMID: 12393605.

28. Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med. 1994; 331:896–903. PMID: 8078551.
29. Fukushima T, Urasaki Y, Yamaguchi M, et al. A randomized comparison of modified intermediate-dose Ara-C versus high-dose ara-c in post-remission therapy for acute myeloid leukemia. Anticancer Res. 2012; 32:643–647. PMID: 22287757.
30. Löwenberg B, Pabst T, Vellenga E, et al. Cytarabine dose for acute myeloid leukemia. N Engl J Med. 2011; 364:1027–1036. PMID: 21410371.

SUPPLEMENTARY MATERIALS
Supplementary Table 1
Early treatment outcomes according to the intensity of the BHAC-based induction chemotherapy.
Supplementary Table 2
Early treatment outcomes according to the intensity of the cytarabine-based induction chemotherapy.
Fig. 1
Treatment strategy for adult patients with acute myeloid leukemia (AML) at the Catholic Blood and Marrow Transplantation Center in Korea. Abbreviations: BHAC, N4-behenoyl-1-β-D-arabinofburanosyl cytosine; CR, complete remission; D7BM, bone marrow blast evaluation on day 7 of chemotherapy; HCT, hematopoietic cell transplantation; MSD, matched sibling donor; MTZ, mitoxantrone; URD, unrelated donor.
Fig. 2
Early treatment outcomes according to the intensity of induction chemotherapy. (A) Early death rates. (B) Early relapse rates before hematopoietic cell transplantation (HCT). (C) Early death rate among elderly patients (older than 55 yr). (D) Early relapse rate before HCT among patients younger than 55 years.
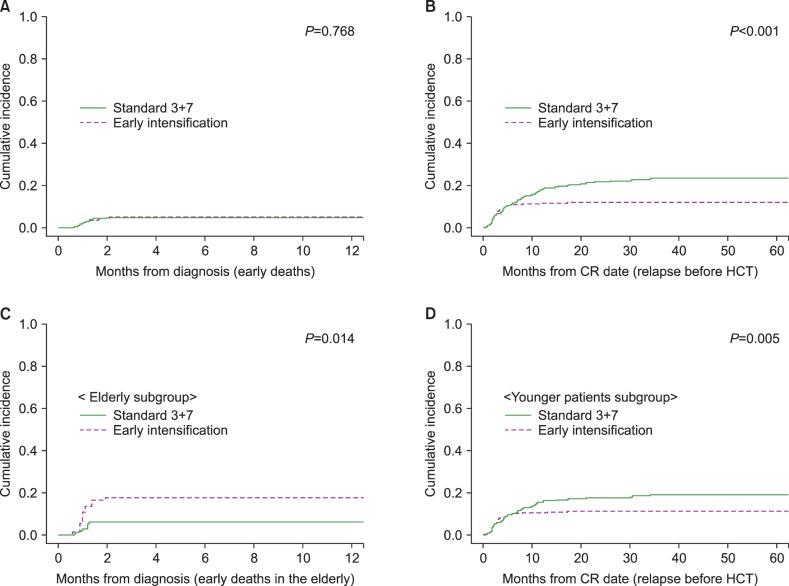
Fig. 3
Overall survival (OS) and overall relapse rates according to the intensity of induction chemotherapy. (A) OS. (B) Overall cumulative incidence of relapse. (C) OS in the elderly patients (older than 55 yr). (D) OS in patients younger than 55 years.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download