Introduction
The first successful cord blood transplant (CBT) was performed about 30 years ago. Nowadays, worldwide public cord blood banks (CBBs) that store about 700,000 units of cord blood release 3,000–4,000 units of unrelated CBs annually for use in stem cell transplantation (SCT). Only 4–5% of stored CBs are used for SCT. Recent Korean and Japanese data revealed that the utilization rates of stored public CBs were higher for units with high total nucleated cell (TNC) counts [1]. In addition to public CBBs, there are over 200 private CBBs located in 54 countries, and as of December 1, 2014, an estimated 4.03 million CB units were stored in private banks worldwide. Based on survey responses from 59 private CBBs, we identified 1,015 case reports of CBTs, of which 530 were devoted to autologous and 485 to allogeneic SCTs as of the end of 2013 [2]. The current utilization rate of public and private CBs is still low. Therefore, strategies for sustaining the profitable future of public and private CBBs have been proposed based on utilization rates and utilization scores. In previous papers I emphasized that the ability to store higher cell doses of CB must be in place before the utilization rate of banked CBs can be enhanced. Furthermore, reductions in the release costs for CB units would improve the use of banked CB. Private CBs and some stored public CBs with lower cell doses might be released for various cell therapies.
Current clinical trials
CB mononuclear cells: Clinical trials in non-transplant settings have already been taking place using CB mononuclear cells (MNCs) for patients with cerebral palsy [3], autism, neonatal hypoxic ischemic encephalopathy, type 1 diabetes mellitus (T1DM), and other conditions. Such clinical trials in non-transplant settings could also enhance the utilization of stored CBs.
Ex vivo expansion of CB: Results of clinical trials using traditional methods of CB expansion with cytokines alone were disappointing, although a number of in vitro studies have reported positive expansion data. However, recently, clinical grade expansions of CB cells were attempted with a variety of techniques: by blocking in vitro differentiation of early progenitor cells using copper chelator, nicotinamide analog, or aryl hydrocarbon receptor antagonist; by inducing constitutive Notch signaling; and by co-culturing CB cells with mesenchymal stem cells (MSCs). Some of these clinical studies expanded a fraction of a CB unit in the setting of single-unit CBT, while others did so in the setting of double unit CBT. Some authors used an unselected CB cell population for expansion, while others used a selected subset such as CD34+ or CD133+ cells. So far, more than 20 clinical studies using CB expansion products have been performed.
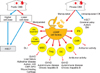
Immunotherapeutic approaches: A recent surge in studies of CB cell subpopulations and advances in ex vivo culture technology have expanded the potential of CB to generate cellular products. A number of immunotherapeutic approaches have been made to enhance the outcomes of CBT, including donor lymphocyte infusion (DLI), the expansion of MSCs, the generation of antigen-specific T cells from CB, re-directing CBT cells using chimeric antigen receptors (CAR), and generating CB-derived natural killer (NK) cells and regulatory T cells (Tregs) (Fig. 1). There have been no attempts to use DLI for relapsed hematologic malignancies in CBT settings, but Berglund et al. [4] recently performed a preliminary safety study using cultured CB T cells for DLI after CBT. CB-derived MSCs have been used for the prevention and treatment of graft versus host disease (GVHD). Chen et al. [5] conducted a systematic review and meta-analysis of published clinical trials to determine the efficacy of MSCs for treating steroid-refractory acute GVHD (aGVHD). Younger age, skin involvement, lower aGVHD grade, and the number of infusions were the main prognostic factors affecting the efficacy of MSC therapy for steroid-refractory aGVHD.
Tregs from CB have been used for the prevention of GVHD. Brunstein reported the results of the first human clinical trial of CB-derived CD4+/CD25+ Tregs in the setting of CBT [6]. In that study, hematopoietic recovery and chimerism, cumulative density of infections, nonrelapse mortality, relapse, and disease-free survival were similar in Treg recipients and controls. KT64/86-expanded CB Tregs were safe and resulted in a low risk of aGVHD. However, low yield prevented further dose escalation. Therefore, another study aimed to develop current good manufacturing practice (cGMP) purification and expansion of CB-Treg cells to optimize yield [7]. In non-transplant settings, Tregs also have been applied to treat autoimmune diseases such as T1DM. Defects in the frequency and function of Treg cells resulted in impaired immunoregulation of autoreactive T cells that destroyed pancreatic islet β-cells, contributing to the onset and development of T1DM. Therefore, therapies designed to stimulate the activity and generation or proliferation of Treg may be helpful for patients with T1DM. Tregs administration also resulted in lower requirements for exogenous insulin, with 2 children in one study becoming completely insulin independent at 1 year [8].
Although numerous studies have been conducted using peripheral blood- or bone marrow-derived NK cell infusions, no clinical study to date has addressed the use CB NK cells. A handful of clinical trials are currently underway to evaluate the feasibility, safety, and efficacy of CB NK cell adoptive immunotherapy in transplant and non-transplant settings [9]. CAR-T/NK cell therapy is a type of cellular therapy that redirects a patient's T/NK cells to specifically target and destroy tumor cells. Clinical trials using CB-derived CAR-engineered NK cells for B lymphoid malignancies are ongoing. Dendritic cells (DCs) are also important to maintain immune tolerance through central and peripheral tolerance mechanisms. The potential roles of such DCs in autoimmune diseases such as T1DM have also been investigated. In such studies, researchers observed decreased DC numbers but increased interferon-α production in first-degree relatives of T1DM patients, which suggests that DCs could be applied to treat T1DM [10]. New advances in the derivation of cGMP-grade human induced pluripotent stem cells (iPSCs) from CB and advances in differentiation protocols have provided bases for converting CB into iPSCs for cell-based therapeutics to treat diseases that remain incurable.
Future perspectives
Because cellular products are in high demand in research and clinical settings, public and private CBBs that store immediately-available clinical-grade cell sources could extend the applicability of CBs by producing various cellular products (Fig. 2). CBBs must establish cGMP facilities in order to prepare for these cellular products derived from CBs. CBBs should then actively collaborate with clinicians conducting pre-clinical and clinical studies using the CB-derived cellullar resources. Then, publically or privately stored CBs (particularly samples with low cell doses) except for those reserved for SCT should be utilized for clinical trials in transplant settings, as well as in non-transplant settings such as treatment of autoimmune diseases and neurodegenerative diseases. Finally, better alignment of laws and regulations governing CB banking to match Food and Drug Administration regulations and guidance for cell therapeutics is necessary.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download