Abstract
Background
Childhood immune thrombocytopenic purpura (ITP) is a common acquired bleeding disorder. Even though most children recover, either spontaneously or with therapy, 10-20% of newly diagnosed ITP cases have a chronic course beyond 12 months. This study evaluated whether clinical and laboratory findings can predict the response to intravenous immunoglobulin (IVIG) and progression to persistent or chronic ITP in children.
Methods
During the period between March 2003 and June 2015, we retrospectively analyzed 72 children, newly diagnosed with ITP, who received IVIG treatment. Peripheral blood counts were obtained at diagnosis and at 1, 3, 6, and 12 months after IVIG treatment.
Results
After 6 months of IVIG treatment, 14 of 72 patients (19.4%) had persistent ITP, and after 12 months, 7 of 40 patients (17.5%) had chronic ITP. Age at diagnosis, gender, history of viral infection, or vaccination before disease onset were not statistically correlated with platelet recovery at 6 and 12 months. However, a platelet count recovery of ≥100×103/µL at 1 and 3 months was significantly correlated with platelet recovery at 6 (P<0.001 and P<0.001, respectively) and 12 (P=0.007 and P=0.004, respectively) months.
Childhood immune thrombocytopenic purpura (ITP) is a common acquired bleeding disorder that is characterized by isolated thrombocytopenia (peripheral blood platelet count <100×103/µL) in the absence of other causes of thrombocytopenia [1]. It is an autoimmune-mediated condition that results from antibody-mediated destruction of platelets and impaired megakaryocyte platelet production [2]. Newly diagnosed ITP may follow within a few weeks after an antigenic challenge such as infection or vaccination [3].
Management of newly diagnosed ITP consists of careful observation, regardless of platelet count [145]. Severe bleeding, which occurs in only 3%–5% of children, requires treatment with corticosteroids, intravenous immunoglobulin (IVIG), or anti-Rhesus-D immunoglobulin [16]. Imbach et al. [7] and Bussel and Hilgartner [8] proposed high-dose IVIG treatment (0.8–1 g/kg) and reported a success rate of 80%. This strategy results in more rapid increases in platelet counts than treatment with oral steroid therapy [9].
Most children with ITP have an acute presentation of purpura and bruising, and 80%–90% of the cases recover spontaneously or with therapy [13]. However, in 10–20% of newly diagnosed children, ITP has a chronic course that persists beyond 12 months [510]. Because of the high impact of ITP on a child's everyday life and activities, identification of prognostic factors would be beneficial for reducing stress and improving quality of life for both these children and their parents [1112].
The aim of this study was to evaluate whether clinical and laboratory findings can predict the response to IVIG treatment and the progression of the condition to persistent or chronic ITP in children.
We retrospectively reviewed the clinical data of newly diagnosed ITP patients (<18 yr) who were given IVIG as initial treatment at Seoul National University Bundang Hospital from December 2003 to May 2015. A total of 123 medical records were available, providing demographic data, the history of antecedent infection or vaccination, initial and follow-up platelet counts, platelet responses to IVIG treatment, and the final outcome from the time of initial diagnosis. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No B-1407/260-103). Informed consent was waived by the board.
Complete response (CR) was defined as a platelet count of ≥100×103/µL. Response (R) to treatment was defined as a platelet count of ≥30×103/µL and a ≥2-fold increase from the baseline count as well as absence of bleeding [13]. Loss of CR or R was defined as a platelet count of <100×103/µL or bleeding (for CR) or platelet count of <30×103/µL or a <2-fold increase from the baseline platelet count or bleeding (for R) [13]. Chronic ITP was defined as thrombocytopenia persisting for longer than 12 months after the initial diagnosis [13]. Patients who did not achieve a platelet count of ≥100×103/µL at 6 months were categorized as persistent ITP.
IVIG was given as the initial treatment to patients with platelet counts of <20×103/µL or those with signs of bleeding at the time of diagnosis of ITP. IVIG was administered at a dose of 1 g/kg/day for 1 or 2 days. The IVIG product used for the treatment was I.V.-Globulin SN (Green Cross Corp, Yongin, Korea) or Liv-Gamma (SK Plasma, Seongnam, Korea). Peripheral blood counts were obtained at diagnosis and at 1, 3, 6, and 12 months after IVIG treatment.
SPSS version 20 (IBM Corp., Armonk, NY, USA) was used for data analyses. All values are expressed as median (range). The Mann–Whitney test was used for nonparametric analysis of continuous variables among the study groups. Fisher's exact test was used to compare the distribution of proportional data. Multiple regression analysis was used to evaluate the factors related to progression of the condition to chronic ITP. The level of significance was set at P <0.05.
Among 123 ITP patients newly diagnosed during the study period, 72 (43 male and 29 female) patients, who were followed up for at least 6 months after IVIG treatment, were included in this analysis (Table 1). Forty patients were followed up for more than 12 months. The median age at diagnosis was 25 months (range, 1–168 mo), with 67 patients (93.1%) younger than 8 years. The median platelet count at diagnosis was 8×103/µL (range, 1–49×103/µL). There was a history of viral infection or vaccination within 4 weeks before the onset of ITP in 55 (76.4%) and in 13 (18.1%) of the 72 patients, respectively.
Among 72 patients, 66 received 1 g/kg/day of IVIG for 2 days and 6 received this dose for 1 day because of side effects or a platelet recovery of ≥50×103/µL after 1 dose of IVIG. Four patients who did not show a response to IVIG in 3 days received corticosteroid (2 mg/kg/day) for 7–14 days as an adjunctive treatment to IVIG, and 1 patient with intracranial hemorrhage received methylprednisolone (30 mg/kg/day) for 3 days as well as a platelet transfusion.
The CR or R within 7 days of IVIG were observed in 68 patients (94.4%). The median times for the platelet count to reach ≥30×103/µL, ≥50×103/µL, and ≥100×103/µL were 2 (range, 1–26), 2 (range, 1–29), and 4 (range, 1–80) days, respectively. Loss of CR occurred in 26 patients after complete recovery of the platelet count in a median of 52 (range, 17–98) days after the initial treatment and a median of 51 (range, 9–92) days after CR.
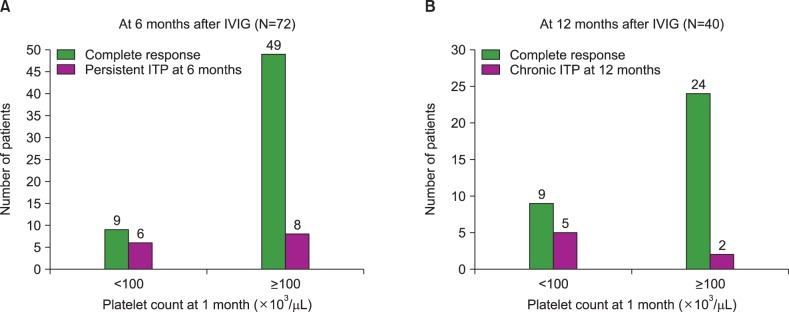
At 6 months of IVIG treatment, 14 of the 72 patients (19.4%) had persistent ITP with platelet counts of <100×103/µL (Table 2). Patient gender, age at diagnosis, a history of viral infection or vaccination before disease onset, an abrupt or insidious onset, the dose of IVIG, the presence of antiplatelet antibodies, and a platelet count of ≥50×103/µL on day 3 of IVIG treatment were not statistically correlated with platelet recovery to ≥100×103/µL at 6 months of IVIG. Furthermore, hemoglobin count, white blood cell (WBC) count, absolute neutrophil count (ANC), and absolute lymphocyte count at diagnosis were not correlated with patient outcome. However, a platelet count of ≥100×103/µL at 1 or 3 months after IVIG treatment was significantly correlated with platelet recovery at 6 months of IVIG (both P <0.001) in univariate analysis. Among the 15 patients with platelet counts of <100×103/µL at 1 month of IVIG treatment, 6 (40%) had persistent ITP at 6 months of IVIG, whereas 8 of 57 patients (14%) with a platelet count of ≥100×103/µL at 1 month had persistent ITP at 6 months of IVIG (P=0.003; Fig. 1A).
At 12 months of IVIG treatment, 7 of 40 patients (17.5%) had chronic ITP, with platelet counts of <100×103/µL (Table 3). Patient gender, age at diagnosis, a history of viral infection or vaccination before disease onset, an abrupt or insidious onset, the dose of IVIG, the presence of antiplatelet antibodies, and a platelet count of ≥50×103/µL on day 3 of IVIG treatment were not statistically correlated with platelet recovery to ≥100×103/µL at 12 months after treatment. In univariate analysis, hemoglobin levels at the time of diagnosis (P=0.034), WBC count at 1 month (P=0.049), and ANC at 1 month (P=0.025) were significantly lower in the CR group (N=33). In addition, platelet counts at 1 month (P=0.007) and 3 months (P=0.004) were significantly lower in the chronic ITP group (N=7).
In multivariate analysis, only the platelet count at 1 month of treatment was related to platelet recovery at 12 months, with borderline significance (P=0.621). Among 14 patients with a platelet count of <100×103/µL at 1 month of IVIG treatment, 5 (35.7%) had chronic ITP at 12 months of treatment, whereas only 2 of 26 patients (7.7%) with a platelet count of ≥100×103/µL at 1 month of treatment had chronic ITP at this time point (P=0.039; Fig. 1B).
It is important to identify reliable predictors of the outcome of childhood ITP at the time of diagnosis as well as after the initial therapy. This would help clinicians to provide patients and their parents with specific information about the expected clinical course and could guide the decision on therapeutic management of the disease [1112].
Many efforts have been made to identify patients at risk of developing persistent or chronic ITP [1415161718192021]. A review of the literature suggested several predictors of recovery in children with ITP [1112]. Infants are more likely to have a short duration of the disease, whereas adolescents have a higher chance of developing chronic disease. A prospective study that included 409 children newly diagnosed with ITP defined 6 clinical parameters that are associated with a short duration of the disease: abrupt onset (history of <2 weeks of bleeding), age at onset <10 years, preceding viral infection, a platelet count at diagnosis of <5×103/µL, wet purpura, and male gender [10]. A young age at onset of the disease may also be associated with a better chance of recovery from chronic ITP [20]. Recent results of a systematic review and meta-analysis showed that potential predictors of the course of childhood ITP, such as age, gender, preceding infection, duration of symptoms, bleeding tendency, and platelet count at diagnosis, were indeed supported by a considerable level of evidence [12].
In this study, 14 of 72 patients (19.4%) at 6 months and 7 of 40 patients (17.5%) at 12 months had persistent and chronic ITP, respectively; these findings were comparable to other previous reports. However, known risk factors of chronic ITP, such as the patient's gender, age at diagnosis, a history of viral infection or vaccination before disease onset, an abrupt or insidious onset, and the presence of antiplatelet antibodies were not correlated with a platelet recovery to ≥100×103/µL at 6 and 12 months.
Drug treatment at diagnosis has been reported to be a possible prognostic factor in the development of chronic disease. Yildiz et al. [22] found that recurrence was significantly less in a no-therapy group compared with children treated with corticosteroid or IVIG. In contrast, Tamminga et al. [23] showed that initial treatment with IVIG was associated with a small but definite increase in the chance of recovery of the platelet count at 6 months, independent of other known risk factors [12]. A prospective randomized study is necessary to elucidate the protective effect, and related underlying mechanism, of IVIG. Because only patients initially treated with IVIG were included in this study to ensure a homogenous group, we could not evaluate the protective effect of IVIG in comparison with other treatment options.
There have been some reports predicting outcomes of ITP after IVIG treatment. Morimoto et al. retrospectively evaluated 49 patients with newly diagnosed ITP who were initially treated with IVIG. They found that patients with a WBC count of <7.0×103/µL at the time of diagnosis had a lower probability of thrombocytopenia-free survival and a higher rate of progression to chronic ITP than those with a WBC count of ≥7.0×103/µL [24]. In another retrospective study by Kim et al. [25], the individual response rate of IVIG treatment was a predictor of a prolonged disease course. Among 182 patients followed up over 6 months, a slow response to IVIG (≥3 doses of 400 mg/kg/day or 200 mg/kg/day before a platelet recovery to ≥50×103/µL) was associated with development of persistent or chronic ITP.
In this study, a WBC count at the time of diagnosis and the time to a platelet recovery to ≥50×103/µL after IVIG treatment were not related to the development of persistent or chronic ITP. At 6 months follow-up, only platelet count recoveries at 1 and 3 months of IVIG treatment were significantly related to persistent ITP in univariate analysis. Because other factors did not show statistically significant differences, a multivariate analysis was not performed. At 12 months follow-up, the platelet count recovery at 1 month showed borderline significance in a multivariate analysis. A higher proportion of patients with a low platelet count (<100×103/µL) at 1 month of IVIG treatment developed persistent or chronic ITP compared with patients with a 1-month platelet recovery count of ≥100×103/µL. The significance of low hemoglobin levels, WBC, and ANC at 1 month in univariate analysis was not clear. The small number of patients may have influenced this result.
This study has additional limitations. First, because this was a retrospective study, the interpretation of data may be limited. Second, screening for Helicobacter pylori infection was not performed, although no increase in platelet counts after eradication of H. pylori has been reported in Korean children with chronic ITP [26]. Lastly, genetic biomarkers of childhood chronic ITP, such as VNN-1 [27] and the Q63R missense variant of the gene encoding cannabinoid receptor type 2 [28] were not evaluated in this study. These biomarkers could be useful for direct and early prediction of prognosis.
In conclusion, this study demonstrated that an early platelet count recovery within 3 months of IVIG treatment predicts a short duration of disease and a favorable outcome in children with newly diagnosed ITP. Further investigation in a larger group of patients is warranted to validate these findings. Future studies should investigate the pathophysiological mechanism underlying this association.
ACKNOWLEDGMENTS
We are grateful to Dr. J.B. Lee and S.Y. Ahn of the Division of Statistics of the Medical Research Collaborating Center at Seoul National University Bundang Hospital for statistical analyses.
References
1. Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011; 117:4190–4207. PMID: 21325604.

2. Neunert CE. Current management of immune thrombocytopenia. Hematology Am Soc Hematol Educ Program. 2013; 2013:276–282. PMID: 24319191.

3. Stasi R, Newland AC. ITP: a historical perspective. Br J Haematol. 2011; 153:437–450. PMID: 21466538.

4. Labarque V, Van Geet C. Clinical practice: immune thrombocytopenia in paediatrics. Eur J Pediatr. 2014; 173:163–172. PMID: 24390128.

5. Schultz CL, Mitra N, Schapira MM, Lambert MP. Influence of the American Society of Hematology guidelines on the management of newly diagnosed childhood immune thrombocytopenia. JAMA Pediatr. 2014; 168:e142214. PMID: 25288142.

6. Neunert CE, Buchanan GR, Imbach P, et al. Severe hemorrhage in children with newly diagnosed immune thrombocytopenic purpura. Blood. 2008; 112:4003–4008. PMID: 18698007.

7. Imbach P, Barandun S, d'Apuzzo V, et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981; 1:1228–1231. PMID: 6112565.

8. Bussel JB, Hilgartner MW. The use and mechanism of action of intravenous immunoglobulin in the treatment of immune haematologic disease. Br J Haematol. 1984; 56:1–7. PMID: 6367804.

9. Beck CE, Nathan PC, Parkin PC, Blanchette VS, Macarthur C. Corticosteroids versus intravenous immune globulin for the treatment of acute immune thrombocytopenic purpura in children: a systematic review and meta-analysis of randomized controlled trials. J Pediatr. 2005; 147:521–527. PMID: 16227040.

10. Rosthøj S, Hedlund-Treutiger I, Rajantie J, et al. Duration and morbidity of newly diagnosed idiopathic thrombocytopenic purpura in children: A prospective Nordic study of an unselected cohort. J Pediatr. 2003; 143:302–307. PMID: 14517509.

11. Yacobovich J, Revel-Vilk S, Tamary H. Childhood immune thrombocytopenia--who will spontaneously recover? Semin Hematol. 2013; 50(Suppl 1):S71–S74. PMID: 23664522.

12. Heitink-Pollé KM, Nijsten J, Boonacker CW, de Haas M, Bruin MC. Clinical and laboratory predictors of chronic immune thrombocytopenia in children: a systematic review and meta-analysis. Blood. 2014; 124:3295–3307. PMID: 25305206.

13. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009; 113:2386–2393. PMID: 19005182.

14. Zeller B, Rajantie J, Hedlund-Treutiger I, et al. Childhood idiopathic thrombocytopenic purpura in the Nordic countries: epidemiology and predictors of chronic disease. Acta Paediatr. 2005; 94:178–184. PMID: 15981751.

15. Donato H, Picón A, Martinez M, et al. Demographic data, natural history, and prognostic factors of idiopathic thrombocytopenic purpura in children: a multicentered study from Argentina. Pediatr Blood Cancer. 2009; 52:491–496. PMID: 19058214.

16. Ahmed I, Rajpurkar M, Thomas R, Chitlur M. Initial lymphocyte count and the development of persistent/chronic immune thrombocytopenic purpura. Pediatr Blood Cancer. 2010; 55:508–511. PMID: 20658623.

17. Ho WL, Lee CC, Chen CJ, et al. Clinical features, prognostic factors, and their relationship with antiplatelet antibodies in children with immune thrombocytopenia. J Pediatr Hematol Oncol. 2012; 34:6–12. PMID: 22215094.

18. Kubota M, Adachi S, Usami I, et al. Characterization of chronic idiopathic thrombocytopenic purpura in Japanese children: a retrospective multi-center study. Int J Hematol. 2010; 91:252–257. PMID: 20049564.

19. Deel MD, Kong M, Cross KP, Bertolone SJ. Absolute lymphocyte counts as prognostic indicators for immune thrombocytopenia outcomes in children. Pediatr Blood Cancer. 2013; 60:1967–1974. PMID: 24038723.

20. Revel-Vilk S, Yacobovich J, Frank S, et al. Age and duration of bleeding symptoms at diagnosis best predict resolution of childhood immune thrombocytopenia at 3, 6, and 12 months. J Pediatr. 2013; 163:1335–1339. PMID: 23891349.

21. Evim MS, Baytan B, Güneş AM. Childhood immune thrombocytopenia: long-term follow-up data evaluated by the criteria of the international working group on immune thrombocytopenic purpura. Turk J Haematol. 2014; 31:32–39. PMID: 24764727.

22. Yildiz I, Ozdemir N, Celkan T, et al. Initial management of childhood acute immune thrombocytopenia: single-center experience of 32 years. Pediatr Hematol Oncol. 2015; 32:406–414. PMID: 26154620.

23. Tamminga R, Berchtold W, Bruin M, Buchanan GR, Kühne T. Possible lower rate of chronic ITP after IVIG for acute childhood ITP an analysis from registry I of the Intercontinental Cooperative ITP Study Group (ICIS). Br J Haematol. 2009; 146:180–184. PMID: 19466971.

24. Morimoto Y, Yoshida N, Kawashima N, Matsumoto K, Kato K. Identification of predictive factors for response to intravenous immunoglobulin treatment in children with immune thrombocytopenia. Int J Hematol. 2014; 99:597–602. PMID: 24573984.

25. Kim UH, Lee SI, Lee KS. Early prediction of chronic childhood immune thrombocytopenic purpura according to the response of immunoglobulin treatment. Clin Pediatr Hematol Oncol. 2013; 20:79–85.
26. Park SJ, Baek HJ, Lee SU, et al. Helicobacter pylori infection in children with chronic idiopathic thrombocytopenic purpura. Clin Pediatr Hematol Oncol. 2008; 15:83–91.
27. Zhang B, Lo C, Shen L, et al. The role of vanin-1 and oxidative stress-related pathways in distinguishing acute and chronic pediatric ITP. Blood. 2011; 117:4569–4579. PMID: 21325602.

28. Rossi F, Mancusi S, Bellini G, et al. CNR2 functional variant (Q63R) influences childhood immune thrombocytopenic purpura. Haematologica. 2011; 96:1883–1885. PMID: 21828121.

Fig. 1
Relationship between platelet counts at 1 month and recovery at 6 and 12 months after IVIG treatment. (A) Seventy-two patients were followed up at 6 months after the initial diagnosis of ITP and IVIG treatment. Among 15 patients with platelet <100×103/µL at 1 month after IVIG treatment, 6 patients (40%) had persistent ITP at 6 months, while 8 of 57 patients (14%) with platelet ≥100×103/µL at 1 month had persistent ITP at 6 months (P=0.003). (B) Forty patients were followed up at 12 months after the initial diagnosis and IVIG treatment. Among 14 patients with platelet <100×103/µL at 1 month of IVIG treatment, 5 patients (35.7%) had chronic ITP at 12 months, while only 2 of 26 patients (7.7%) with platelet ≥100×103/µL at 1 month had chronic ITP (P=0.039).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download