Abstract
Heparin-induced thrombocytopenia (HIT) is a serious, immune mediated complication of exposure to unfractionated or low-molecular-weight heparin. Though rare, it is a condition associated with high morbidity and mortality that requires immediate change to alternative anticoagulants for the prevention of life-threatening thrombosis. The direct thrombin inhibitors lepirudin and argatroban are currently licensed for the treatment of HIT. Dabigatran, a novel oral anticoagulant (NOAC) with a similar mechanism of action and effective use in other indications, has recently been proposed as another therapeutic option in cases of HIT. This review serves as an introduction to using dabigatran for this purpose, detailing the clinical aspects of its administration, evidence of its performance compared to other anticoagulants, and the preliminary reports of HIT successfully treated with dabigatran. As the literature on this develops, it will need to include clinical trials that directly evaluate dabigatran against the other NOACs and current treatment options.
Heparin-induced thrombocytopenia (HIT) is an immune mediated, prothrombotic adverse reaction to unfractionated heparin (UFH) and low-molecularweight heparin (LMWH). These heparin products form multimolecular complexes with platelet factor 4 (PF4) which are antigenic, resulting in the formation of IgG platelet activating antibodies against the heparin/PF4 complex. The immune complexes comprised of the heparin/PF4 complexes and the antibodies in turn bind to the FcγIIa (IgG) receptors of platelets, inducing a sequence of platelet activation, platelet aggregation, and procoagulant platelet-derived microparticle generation (Fig. 1). This process ultimately leads to platelet consumption and thrombin generation, thus resulting in thrombocytopenia and a paradoxical risk for venous and arterial thrombosis [12].
HIT is divided into two clinical entities, HIT type I and HIT type II. HIT type I is a benign transient mild thrombocytopenia that occurs early on in heparin therapy and usually resolves with continued exposure. It is non-immune and results from the direct effect of heparin on platelets causing aggregation and splenic sequestration. HIT type I is not associated with thromboembolic events. On the other hand, HIT type II is a severe persistent immune mediated reaction that develops after several days of heparin exposure and may lead to thrombosis, amputation, and even death. Heparin treatment must be discontinued immediately in cases of HIT type II [2]. For the purposes of this review, the term HIT will be used to refer to HIT type II.
Treatment for HIT consists of discontinuing heparin and starting alternative anticoagulation. In the United Kingdom, the alternative anticoagulants licensed for use in HIT are argatroban and danaparoid [3]. In the United States, the only drugs currently approved by the Food and Drug Administration (FDA) for treatment of HIT are argatroban and lepirudin [4].
Dabigatran, the first oral direct thrombin inhibitor (DTI) marketed in the United States, has several advantages as an anticoagulant, including its route of administration, rapid onset of action, predictable and consistent anticoagulation profile, reversibility, and safety. Dabigatran therapy does not require routine coagulation monitoring or dose titration, unlike warfarin. By virtue of its composition and mechanism of action, it does not interact with HIT antibodies and poses no risk of cross-reactivity [5]. It has been shown to be superior and non-inferior to enoxaparin and warfarin in various other applications, including venous thromboembolism (VTE) prophylaxis, acute VTE treatment, and stroke prevention in nonvalvular atrial fibrillation (AF) patients [6]. These characteristics have led to investigations into dabigatran's potential to be a convenient, effective, and well tolerated treatment for HIT.
Current medications used for heparin-induced thrombocytopenia include lepirudin, danaparoid, and argatroban. Lepirudin is a recombinant hirudin, a direct, specific, and irreversible thrombin inhibitor. Its pharmacokinetics depend strongly on renal function and immunogenicity [7]. Lepirudin requires strict laboratory monitoring, especially in patients undergoing cardiopulmonary bypass surgery and those who have impaired renal function. The lack of an antidote to lepirudin can also be a problem in patients on hemofiltration with high-flux filter systems, hemodialysis and plasmapheresis are the only treatments available for hirudin overdose [8]. Lepirudin is no longer used in the US due to reports of anaphylactic reactions with repeated exposure, leading to its withdrawal by the manufacturer in spring 2012, despite retrospective studies concluded that its overall benefit in treating HIT outweighed this risk [910].
Danaparoid is a heparinoid mixture of heparan sulfate, dermatan sulfate, and chondroitin sulfate with predominantly antifactor Xa and some anti-Factor IIa activity [7]. Unique to drugs used for HIT, danaparoid specifically suppresses HIT antibody-induced platelet activation by replacing heparin/PF4 complexes on the platelet surface [11]. Due to its composition, danaparoid can rarely demonstrate cross-reactivity with HIT antibodies associated with high rates of thrombotic complications and mortality [12]. In one large retrospective study, 49% of patients with confirmed cross-reactivity experienced thromboembolic complications with an attributable death rate of 17%. Routine clinical and platelet monitoring is required, and patients with new/persistent platelet count reduction and/or new/extended thrombosis should be switched to an alternative anticoagulant [13]. That being said, the drug is no longer marketed in the US and has suffered manufacturing problems, causing a worldwide shortage in recent years [14].
Argatroban is a synthetic DTI derived from L-arginine that reversibly binds to the thrombin active site in both free and clot-bound thrombin. It prevents fibrin formation, platelet aggregation, and the activation of protein C and coagulation factors V, VIII, and XIII [7]. It binds highly selectively without any co-factors and can be used in patients with isolated renal impairment without dose adjustments [15]. However, its effect requires monitoring and its metabolism is strongly hepatic [14]. Dose reductions and adjustments for critically ill and cardiac surgery patients, as well as those with impaired liver function, are required for its use. Overall, it is considered a safe therapeutic option in these settings [16].
Dabigatran was the first oral direct thrombin inhibitor (DTI) marketed in the United States as well as the first of the group of drugs known as novel or non-vitamin K antagonist oral anticoagulants (NOACs) [1718]. Its prodrug, dabigatran etexilate, was first patented in 1997 [19].
Since 2010, dabigatran has been approved by the U.S. Food and Drug Administartion (FDA) for the prevention of stroke and systemic embolism in patients with nonvalvular AF, treatment of deep venous thrombosis (DVT) and pulmonary embolism (PE) in patients who have been administered a parenteral anticoagulant for five to ten days, reduction of the risk of recurrence of DVT and PE in patients previously treated with other anticoagulants, and prophylaxis of DVT and PE following hip replacement surgery [51720]. In Europe, dabigatran has been licensed for VTE prevention in adult patients undergoing elective total hip or knee replacement since 2008 [1921].
Dabigatran is a reversible, competitive inhibitor of thrombin, to which it binds in both freely circulating and clotbound forms. Like other NOACs, its mechanism of action does not interfere with the interaction of platelets and PF4 or that of antibodies with the heparin/PF4 complex [21].
Dabigatran etexilate undergoes liver hydrolylation to dabigatran, its active metabolite. While a study showed that bioconversion is slower in patients with hepatic impairment, overall this observation does not significantly affect the safety profile of dabigatran [22]. The manufacturer accordingly recommends no dose adjustment in patients with moderate hepatic impairment [20]. However, the European Medicines Agency (EMA) summary of product characteristics advises against the use of dabigatran in patients with hepatic impairment or liver disease expected to have any impact on survival [23].
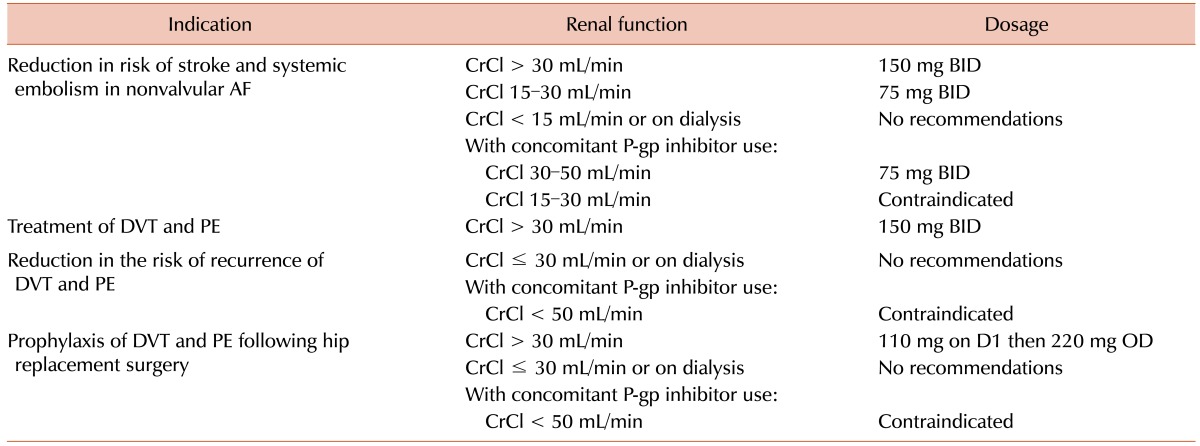
Excretion of dabigatran, on the other hand, is predominantly renal (approximately 80%, much higher than that of all other NOACs to date) [2425]. Accordingly, there are guidelines for dose adjustment in patients with decreased creatinine clearance (CrCl) [182026]. The 2015 United States dosing recommendations (Table 1) state that in patients with creatinine clearance less than 30 mL/min—i.e., those with severe renal impairment or stage IV chronic kidney disease—dabigatran is contraindicated for VTE treatment, reduction of VTE recurrence risk, and VTE prophylaxis following hip replacement. However, in patients with creatinine clearance 15–30 mL/min, it may still be used in non-valvular AF at 75 mg twice daily (BID), half the standard dose of 150 mg BID. This reduced dose is also recommended for patients with CrCl 30–49 mL/min and concomitant use of P-glycoprotein inhibitors, such as verapamil, dronedarone, and systemic ketoconazole [20]. The EMA prescribing guidelines do not recommend dabigatran for any use in patients with CrCl less than 30 mL/min. The European Society of Cardiology guidelines advise that physicians consider a prescription of 110 mg BID rather than 150 mg BID in patients with the following: age ≥80, concomitant use of interacting drugs, HAS-BLED score ≥3, or CrCL 30–49 mL/min [2327].
Advanced hepatic and renal impairment thus have implications for plasma and serum dabigatran concentration. Dabigatran, like other NOACs, does not require routine laboratory monitoring [18]. However, assessment of its anticoagulant effect may be warranted in emergent situations or cases where inordinately high or low drug levels are suspected [28]. Hemoclot thrombin inhibitor, dilute thrombin time (dTT), and ecarin clotting time (ECT) allow for quantification of dabigatran levels, but these assays are not widely available [2930]. Studies have found that activated partial thromboplastin time (aPTT) has a curvilinear relationship with dabigatran. A prolonged aPTT suggests therapeutic or supratherapeutic levels and may be reliably used for semi-quantitative monitoring; a normal aPTT does not exclude the presence of dabigatran [263132]. However, aPTT reagents from different laboratories vary widely in their sensitivity to dabigatran and should be calibrated for such use. PT/INR is insufficiently sensitive to dabigatran and should not be used in the evaluation of its anticoagulant effect [3133]. The standard thrombin time (TT) is oversensitive to dabigatran and is best used to determine only the presence or absence of residual drug [3034]. Overall, aPTT, Hemoclot thrombin inhibitor, and ECT are considered the most appropriate tests for assessing the anticoagulant effect of dabigatran [262835].
Likewise, under certain emergent circumstances the reversal of dabigatran is desirable. The specific antidote idarucizumab, a monoclonal antibody fragment, has recently been approved by the FDA. Idarucizumab binds dabigatran with an affinity 350 times that of the binding between dabigatran and thrombin; the idarucizumab-dabigatran complexes, which are unable to bind thrombin, are cleared by the kidneys. The agent has been found to normalize dTT, ECT, aPTT, and TT within minutes in a dose-dependent fashion [3637]. In phase I trials with healthy volunteers, idarucizumab was found to achieve rapid, complete, and sustained reversal of dabigatran-induced anticoagulation without significant adverse effects [38]. Additionally, in the absence of dabigatran, idarucizumab has no effect on coagulation parameters or endogenous thrombin potential [39]. The initial findings of the phase III Reversal Effects of Idarucizumab on Active Dabigatran (RE-VERSE AD) study show that 5 g of intravenous idarucizumab was sufficient to completely reverse anticoagulation within minutes in 88– 98% of patients. There were no safety concerns among this preliminary cohort, which consisted of 90 patients who either had serious bleeding or required an urgent procedure [40]. The European Heart Rhythm Association recommends 5 g of intravenous idarucizumab for cases of life-threatening bleeding or intracranial hemorrhage secondary to dabigatran anticoagulation [26]. There is some data suggesting that dabigatran may be reinitiated 24 hours after reversal with idarucizumab without altering the level of anticoagulation achieved. Re-exposure to idarucizumab appears to be safe and equally effective as first time treatment [41].
In cases where idarucizumab is not available, activated charcoal may help to reduce continued absorption in cases of overdose if administered within two to three hours of ingestion. Dabigatran is dialyzable due to its low protein binding, making hemodialysis another feasible treatment; however, dialysis is challenging in cases with hemodynamic instability [42]. Prothrombin complex concentrate (PCC) and activated PCC, though not licensed for this usage, are capable of reversing dabigatran-induced anticoagulation in a dose-dependent manner. However, they have no significant effect on aPTT or levels of dabigatran and there are at present no established dosing strategies for this purpose [4344].
Dabigatran has been studied in four large randomized controlled trials comparing it to warfarin, all of which concluded that dabigatran is noninferior and is associated with a lower risk of bleeding.
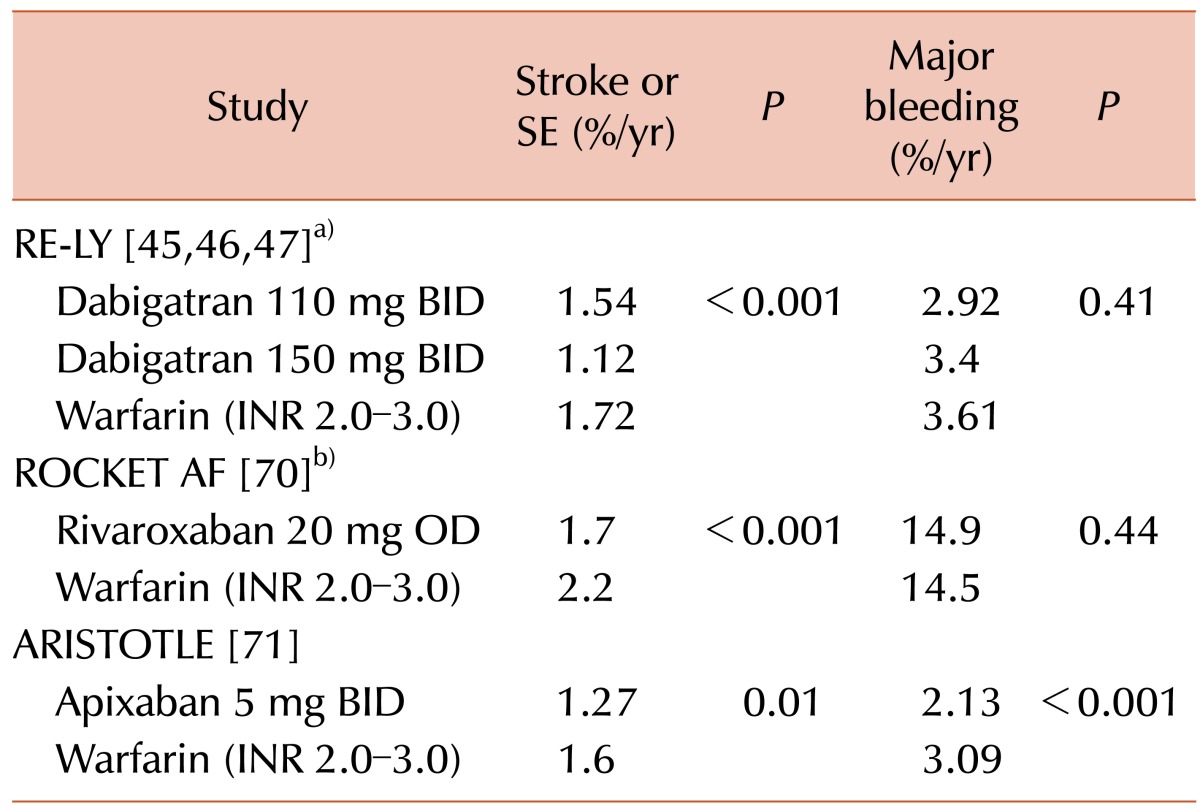
In the RE-LY trial by Connolly et al. [45], 18,113 patients with AF at risk for stroke were randomly assigned to receive dabigatran 110 mg BID, dabigatran 150 mg BID, or dose-adjusted warfarin (INR goal 2.0–3.0). In comparison with warfarin, dabigatran 110 mg BID was not inferior in terms of stroke and systemic embolism rates, as well as superior in risk for major hemorrhage. Dabigatran 150 mg BID was superior to warfarin with respect to the primary outcomes of stroke and systemic embolism, but had similar rates of major hemorrhage. There was no significant higher risk of acute coronary syndromes with dabigatran. Later re-evaluations of the database led to the identification of additional adverse events as well as events previously not reported due to their association with a death. Inclusion of these newly identified events did not alter the conclusions of the study [4647]. The RE-LY trial demonstrated that the benefits of dabigatran over warfarin were similar in hypertensive and non-hypertensive patients [48]; another, that these benefits were greater in diabetic patients compared to nondiabetics [49]. A third analysis found that decline in renal function, which occurred in all treatment groups, was greater in patients taking warfarin versus those assigned to dabigatran [50].
In the two-part RE-COVER study conducted by Schulman et al., the efficacy and safety of dabigatran versus warfarin in the treatment of acute VTE—including both DVT and PE—was evaluated in 2,564 patients. In part one of the trial, subjects received either dabigatran 150 mg BID or dose-adjusted warfarin (INR goal 2.0–3.0) for six months after initial therapy with parenteral anticoagulation. The results demonstrated that dabigatran is not inferior to dose-adjusted warfarin in the prevention of recurrent or fatal VTE. Safety outcomes were also similar between the two drugs. Notably, the incidences of major and minor bleedings were reduced with dabigatran [51]. In the RE-COVER II, 2,589 patients initially received parenteral anticoagulation with warfarin or warfarin-placebo. Afterward, patients received either dabigatran 150 mg BID with a warfarin placebo or warfarin (INR goal 2.0–3.0) with a dabigatran placebo. Six months of intervention produced results that confirmed those of RE-COVER I; dabigatran was as effective as warfarin for treatment of acute VTE, with a lower risk of bleeding and no requirement for laboratory monitoring [52].
Schulman et al. [53] went on to conduct two more trials, which compared dabigatran 150 mg BID with warfarin (RE-MEDY, active-control study) and placebo (RE-SONATE, placebo-control study) in patients with VTE who had already completed at least three months of initial therapy. In the active-control study, dabigatran was not inferior to warfarin for the prevention of VTE, once again with a lower risk of bleeding. In the placebo-control study, dabigatran significantly reduced the rate of recurrent VTE but also showed a significantly higher risk of bleeding. The benefit of treatment with dabigatran was maintained during extended follow-up after the study drug was discontinued. The authors noted that the efficacy of dabigatran and its risk of clinically relevant bleeding versus placebo is similar to that of rivaroxaban compared to placebo. As in the RE-LY trial, there was a higher rate of acute coronary events with dabigatran than warfarin.
There are several reported cases of dabigatran being used for HIT (Table 2). A 67-year-old female with a femoral fracture who developed DVT despite the use of enoxaparin prophylaxis and a platelet count decrease from 173×109/L to 32×109/L. Dabigatran 110 mg BID was initiated and improved the platelet count in a few days to 236×109/L. The patient's thrombus recanalized without complications [54].
Another reported case was a 67-year-old female with a history of AF who received heparin for portal vein thrombosis secondary to essential thrombocythemia. Seven days after the initiation of heparin, the patient's platelet count had decreased from 1,000×109/L to 120×109/L. She was found positive for heparin/PF4 antibodies and also developed a pulmonary embolism. The patient was initially started on lepirudin but after 36 hours was switched to dabigatran. The platelet count doubled after 48 hours of dabigatran therapy, followed by resolution of the pulmonary emboli [55].
Fieland and Taylor [56]. contributed the case of a 70-year-old male with a history of paroxysmal AF who underwent a coronary artery bypass grafting (CABG) procedure. The patient developed AF on post-operative day two and was started on dabigatran 150 mg BID on post-operative day four. Also on post-operative day four, heparin/PF4 antibodies came back positive. The patient was transitioned from dabigatran to warfarin on post-operative day eight. At outpatient follow-up, there were no complaints of bleeding or thrombosis. While dabigatran was given for the FDA-approved use of AF anticoagulation, it also proved useful for HIT therapy in this case.
Finally, Bircan and Alanoglu recently presented the case of a 57-year-old female who received prophylactic enoxaparin for unilateral total knee replacement. She had normal platelet counts of 313×109/L before and 181×109/L after surgery. On day 11 of prophylaxis (post-operative day five), the patient's platelet count had decreased by more than 50%. She was placed on warfarin in addition to enoxaparin. The next day, her platelet count was 66×109/L, liver function tests were elevated, and pulmonary CT angiography confirmed massive PE with bilateral widespread thrombi. Doppler ultrasound revealed an acute thrombus at the right popliteal vein. Testing for heparin/PF4 antibodies was not available. Enoxaparin and warfarin were discontinued immediately and the patient was started on dabigatran 150 mg BID. Over the next few days, platelet counts increased and liver function tests normalized. When the platelet count reached 150×109/L, the patient was switched to warfarin therapy. Once therapeutic INR had been achieved for two days, dabigatran was stopped. The patient was found to have prothrombin 20210A and methylene-tetrahydrofolate reductase C677T heterozygous mutations. She was discharged with recommendations of lifelong folic acid replacement and anticoagulant therapy [57].
The NOAC rivaroxaban, which is an orally administered direct Factor Xa inhibitor, has also showed promise as a potential treatment for HIT. An in vitro study in 2008 by Walenga et al. [58] first showed that rivaroxaban, unlike UFH and LMWH, does not cause platelet activation or aggregation in the presence of HIT antibodies, induce platelets to release PF4, or interact with PF4 that is present.
Since then, rivaroxaban has become the best studied of the NOACs for HIT treatment, with several documented experiences of successful use (Table 2). One case series by Ng et al. [59] detailed three incidences of HIT managed with rivaroxaban in Singapore, where argatroban, danaparoid, and fondaparinux are not registered for use. In case one, the patient developed HIT with thrombosis following heparin exposure during hemodialysis. In case two, the patient developed HIT with postoperative prophylaxis following stenting and arterial bypass. The third patient developed HIT after receiving enoxaparin for the treatment of a pulmonary embolus. In each case, the diagnosis of HIT was confirmed by the presence of heparin/PF4 antibodies. Rivaroxaban was initiated with platelet counts of 69×109/L, 20×109/L, and 28×109/L, respectively. Patients 2o and 3 received extended treatment with rivaroxaban and patient 1 was eventually transitioned to warfarin due to hemodialysis. All three patients experienced no thrombotic or bleeding complications during or following rivaroxaban therapy at follow up of one to two weeks.
Another successful use of rivaroxaban in HIT with thrombosis was reported by Hantson et al. [60] in the case of a 36-year-old man who underwent orthopaedic surgery four days following a traumatic fall. Platelet count at admission was 166×109/L and increased to 280×109/L ,postoperatively. The patient was placed on anticoagulant prophylaxis with nadroparin and on day nine of therapy developed thrombocytopenia, with the platelet count reaching a nadir of 25×109/L by day 12. When the patient tested positive for heparin/PF4 antibodies, nadroparin was replaced by fondaparinux. The patient developed an acute radial artery thrombosis three days later; a diagnosis of HIT-induced thrombosis was made and rivaroxaban 15 mg BID was initiated. The platelet count began to increase 4 days after rivaroxaban was started and normalized at day 10. Partial recanalization of the arterial thrombosis was demonstrated at follow up one and two months after discharge.
Sartori et al. [61] described a 68-year-old male who developed HIT while receiving enoxaparin for an isolated distal DVT in the internal gastrocnemius and soleal veins. Six days after initiation of enoxaparin, the patient's platelet count had decreased from 263×109/L postoperatively to 161×109/L. Fondaparinux was substituted for enoxaparin then two days later, with a confirmed diagnosis of HIT, rivaroxaban 20 mg once daily (OD) was started. The patient's platelet count returned to baseline six days after enoxaparin was discontinued. At follow up three months later, rivaroxaban was discontinued and the DVT had completely recanalized. Testing for heparin/PF4 complexes was negative at six months follow up and the patient had no recurrent thrombotic events or bleeding complications.
Abouchakra et al. [62] submitted the case of a 53-year-old male who developed HIT secondary to cardiac catheterization and CABG. He returned to the hospital with decreased platelets and tested positive for heparin/PF4 antibodies. Repeated cardiac catheterization showed a thrombotic image in the saphenous vein graft-right coronary artery graft and duplex ultrasound demonstrated a fresh mural thrombus of the carotid bulb with 40% stenosis. Due to the lack of lepirudin availability, the patient was started on rivaroxaban and his platelet count gradually recovered. A month later, the thrombotic image had disappeared and the carotid bulb thrombus had faded with no reported bleeding complications.
There was one case, reported by Tardy-Poncet et al. [63], of low-dose rivaroxaban failing to improve thrombocytopenia in a 71-year-old woman with a baseline platelet count of 239×109/L who received unfractionated heparin during perioperative Cell Saver blood collection for autotransfusion. She was not given heparin preoperatively or postoperatively for total knee replacement, though she had previously received low-molecular-weight heparin years earlier for two other surgical procedures. Rivaroxaban 10 mg OD was administered postoperatively but the patient's platelet count continued to decrease to 58×109/L on day 20. On day 21, the patient was switched to dabigatran 220 mg OD. Her platelet count had improved to 150×109/L by day 35 of admission. The rest of her course remained uneventful and the patient was discharged. In vitro studies performed later showed progressively less thrombin generation with increasing rivaroxaban concentrations, suggesting that higher, therapeutic doses are required for adequate anticoagulation in HIT.
A prospective, multicenter, single-arm cohort study on rivaroxaban for HIT has recently concluded and been accepted for publication. This study by Linkins et al. [64] evaluated the incidence of new symptomatic venous and arterial thromboembolism in patients with suspected or confirmed HIT treated with rivaroxaban. The original study design planned for 200 participants to be given different regimens based on whether they had confirmed HIT with or without thrombosis. The final cohort—consisting of 22 patients without thrombosis who presented with an intermediate or high 4Ts pretest probability—received rivaroxaban 15 mg BID until a local HIT assay result was available. Patients with HIT confirmed via serotonin-release assay then continued the course of rivaroxaban until platelet count recovery to either 150×109/L or a lower individual baseline. Once thrombocytopenia was resolved, the participants stepped down to rivaroxaban 20 mg OD until day 30 of the study. At the end of the study, one HIT-positive patient had confirmed new thromboembolism (4.5%; 95% CI, 0– 23.5%) and another HIT-positive participant required limb amputation despite limb recovery. Nine out of the ten HIT-positive patients achieved platelet recovery with a mean time to recovery of 11 days, with one patient taken off rivaroxaban after two doses due to a rise in liver enzymes. No thrombotic or major bleeding events occurred amongst either the HIT-positive or HIT-negative groups [65].
Apixaban, another direct Factor Xa inhibitor, has had more limited evaluation as an anticoagulant for the management of HIT. There has been an in vitro study by Walenga et al. [66] which tested the effect of apixaban on platelets using the serotonin release assay and a platelet aggregation assay. The results of both assays showed a consistent absence of platelet activation and HIT antibody-mediated platelet aggregation by apixaban.
In a 72-year-old woman with lung cancer who developed pulmonary embolism after cerebral metastasectomy, lobectomy, and chemotherapy, ddalteparin was initiated; twenty days later, the patient's platelet count had reduced to 84×109/L. Dalteparin was then discontinued while HIT testing was in progress, due to the limited availability of alternative drugs and the patient's stable condition. The screening test for heparin/PF4 antibodies was highly positive and heparin-induced platelet activation confirmed the diagnosis of HIT. The correct dose of fondaparinux was not available. Furthermore, administration and monitoring of traditional drugs used to manage HIT would have been challenging due to the patient's remote residence. Thus, the patient was placed on apixaban 5 mg BID. Platelet counts and parameters quickly improved and D-dimer decreased. Four weeks later, HIT antibodies remained positive. The six month follow up was pending at the time of publication with no adverse reactions reported thus far [67].
Finally, Sharifi et al. [68] conducted a combined, open label, single arm study of 22 patients with HIT who were treated with different NOACs after a short course of argatroban. Of the cohort, six received dabigatran 150 mg BID; eleven, rivaroxaban 20 mg OD; and five, apixaban 5 mg BID. Patients with a glomerular filtration rate of less than 30 mL/min, bleeding tendencies, and recent surgeries were later switched to apixaban. No patients received vitamin K antagonists for anticoagulation and none had thrombosis at presentation. Although none of the cohort developed major or minor bleeding, recurrent VTE, or arterial thrombosis, six patients died within 19 months after discharge. This was in keeping with other reports on the mortality rate of HIT, independent of thrombosis.
Thus far, comparisons between these three NOACs that may guide a conclusion as to which is the best choice in cases of HIT have taken two forms—in vitro testing and metanalyses with indirect comparisons in their use for other indications. One such in vitro study evaluated dabigatran, rivaroxaban, and 2-O, 3-O sulfated heparin (ODSH) in terms of their effects on heparin/PF4 complexes, the interaction of heparin/PF4 antibodies with platelets, and the prevention of antibody formation. The results showed that neither dabigatran and rivaroxaban—similar to the currently approved drugs for HIT argatroban and lepirudin—had any effect on the interaction of PF4 or heparin/PF4 antibodies with platelets, making them suitable candidates for alternative anticoagulation in patients with a history of HIT. The study also demonstrated that ODSH, when given with UFH or LMWH, prevents formation of immunogenic heparin/PF4 complexes, displaced them and PF4 from the platelet surface, and disrupted these complexes, inhibiting their binding to anti-heparin/PF4 Ab. The low-sulfated heparin performed better with UFH than with LMWH, and a blended UFH-ODSH anticoagulant could potentially prevent the occurrence of HIT [69].
Three phase III randomized clinical trials—dubbed RE-LY [454647], ROCKET AF [70], and ARTISTOTLE [71]—assessed the relative efficacy and safety of dabigatran, rivaroxaban, and apixaban, respectively, compared to warfarin in patients with AF (Table 3). In every trial, the NOACs were found to be at least noninferior to warfarin for the composite endpoint of stroke and systemic embolism. ARISTOTLE and RE-LY demonstrated the superiority of apixaban and dabigatran, respectively, to warfarin with respect to the same endpoint. All three NOACs were associated with decreased risk for hemorrhagic stroke and intracranial bleeding compared to warfarin. Rivaroxaban and dabigatran were found to have risks for major bleeding comparable to that of warfarin, while apixaban was superior in this outcome [457071].
One metanalysis of these trials conducted adjusted indirect comparisons of the three drugs in pairs using warfarin as the common comparator, concluding that dabigatran was associated with a significantly lower risk of the composite of stroke or systemic emboli (RR, 0.76; 95% CI, 0.49–0.93) and ischemic stroke (RR, 0.68; 95% CI, 0.49–0.93) versus rivaroxaban. There was no significant difference in either major bleeding (RR, 0.91; 95% CI, 0.75–1.11) or gastrointestinal bleeding (RR, 0.97; 95% CI, 0.72–1.31) between the agents, whereas dabigatran significantly reduced the risk of hemorrhagic stroke (RR, 0.45; 95% CI, 0.21–0.98) compared with rivaroxaban. When compared with dabigatran, apixaban was slightly but not significantly unfavorable across all efficacy outcomes, including the composite of stroke or systemic emboli (RR, 1.19; 95% CI, 0.90–1.58), ischemic stroke (RR, 1.19; 95% CI, 0.86–1.65), any stroke (RR, 1.21; 95% CI, 0.91–1.62), and mortality (RR, 1.01; 95% CI, 0.86–1.19). Apixaban was associated with a lower risk of major bleeding (RR, 0.75; 95% CI, 0.62–0.92) and particularly gastrointestinal bleeding (RR, 0.60; 95% CI, 0.43–0.84), but there was a significant increase in hemorrhagic stroke (RR, 1.93; 95% CI, 0.93–4.02) with apixaban compared with dabigatran. This study also calculated the absolute differences in events between each pair of drugs. Dabigatran resulted in fewer composite (stroke or systemic emboli) events as well as all other negative outcomes measured versus rivaroxaban, except for gastrointestinal bleedings, of which there were an equal number. Assessed against apixaban, dabigatran was associated with fewer ischemic strokes, nondisabling strokes, deaths, and hemorrhagic strokes, while subjects who received apixaban experienced fewer major and gastrointestinal bleedings [72].
A similar metanalysis performed indirect comparisons of dabigatran 110 mg BID, dabigatran 150 mg BID, rivaroxaban, and apixaban, again using the common comparator warfarin. In terms of efficacy, there was a significantly lower risk of stroke and systemic embolism (by 26%) for dabigatran 150 mg BID compared with rivaroxaban, as well as hemorrhagic stroke and nondisabling stroke. There were no significant differences for apixaban versus dabigatran (both doses) or rivaroxaban. Major bleeding was significantly lower with apixaban compared with dabigatran 150 mg BID (by 26%) and rivaroxaban (by 34%), but not significantly different from dabigatran 110 mg BID. There were no significant differences between apixaban and dabigatran 110 mg BID in safety endpoints. Dabigatran 110 mg BID was associated with less major bleeding (by 23%) and intracranial bleeding (by 54%) as compared to rivaroxaban. There were no significant differences in myocardial infarction events between either dosage of dabigatran and apixaban. Overall, dabigatran 150 mg BID was superior to rivaroxaban for some efficacy endpoints, whereas major bleeding was significantly lower with dabigatran 110 mg BID and apixaban [73].
Dabigatran thus presents a convenient, effective, and safe option for alternative anticoagulation in HIT. It is both non-inferior to warfarin in efficacy and has a lower bleeding risk. In trials assessing its role in stroke prophylaxis for AF patients, dabigatran performs better than other non-vitamin K antagonist oral anticoagulants, particularly with regards to the risk of stroke, systemic embolism, and intracranial bleeding. However, it is associated with a relatively higher risk of gastrointestinal bleeding. It should be noted that dabigatran is already established as an advantageous option in this and other settings, including treatment of VTE, not just prophylaxis. In patients with HIT who require anticoagulation for other indications, dabigatran could serve as both a HIT therapeutic and thrombosis prophylactic.
With the discussed in vitro studies on dabigatran's effect on the pathogenesis of HIT and the clinical trials on dabigatran's uses in other settings done, what remains is the task of performing randomized clinical trials to evaluate the use of dabigatran specifically for the management of HIT. As with the series of trials conducted with the various NOACs, these studies should serve to compare dabigatran head to head with other direct or parenteral thrombin and factor Xa inhibitors, notably argatroban, rivaroxaban, and apixaban. The key concerns with dabigatran that have been raised are its associated risks of gastrointestinal bleeding and acute coronary syndromes, which in turn require more investigation in order to determine their significance.
References
1. Miyares MA, Davis KA. Direct-acting oral anticoagulants as emerging treatment options for heparin-induced thrombocytopenia. Ann Pharmacother. 2015; 49:735–739. PMID: 25855702.

2. Grouzi E. Update on argatroban for the prophylaxis and treatment of heparin-induced thrombocytopenia type II. J Blood Med. 2014; 5:131–141. PMID: 25152637.

3. Watson H, Davidson S, Keeling D. Haemostasis and Thrombosis Task Force of the British Committee for Standards in Haematology. Guidelines on the diagnosis and management of heparininduced thrombocytopenia: second edition. Br J Haematol. 2012; 159:528–540. PMID: 23043677.

4. Linkins LA, Dans AL, Moores LK, et al. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141(Suppl 2):e495S–e530S. PMID: 22315270.
5. Blommel ML, Blommel AL. Dabigatran etexilate: A novel oral direct thrombin inhibitor. Am J Health Syst Pharm. 2011; 68:1506–1519. PMID: 21817082.

6. Pudusseri A, Shameem R, Spyropoulos AC. A new paradigm shift in antithrombotic therapy. Front Pharmacol. 2013; 4:133. PMID: 24155721.

7. Bakchoul T, Greinacher A. Recent advances in the diagnosis and treatment of heparin-induced thrombocytopenia. Ther Adv Hematol. 2012; 3:237–251. PMID: 23606934.

8. Petros S. Lepirudin in the management of patients with heparininduced thrombocytopenia. Biologics. 2008; 2:481–490. PMID: 19707378.

9. Greinacher A, Lubenow N, Eichler P. Anaphylactic and anaphylactoid reactions associated with lepirudin in patients with heparin-induced thrombocytopenia. Circulation. 2003; 108:2062–2065. PMID: 14568897.

10. Cardenas GA, Deitcher SR. Risk of anaphylaxis after reexposure to intravenous lepirudin in patients with current or past heparin-induced thrombocytopenia. Mayo Clin Proc. 2005; 80:491–493. PMID: 15819285.

11. Chong BH, Ismail F, Cade J, Gallus AS, Gordon S, Chesterman CN. Heparin-induced thrombocytopenia: studies with a new low molecular weight heparinoid, Org 10172. Blood. 1989; 73:1592–1596. PMID: 2713496.

12. Tardy-Poncet B, Wolf M, Lasne D, et al. Danaparoid cross-reactivity with heparin-induced thrombocytopenia antibodies: report of 12 cases. Intensive Care Med. 2009; 35:1449–1453. PMID: 19350215.

13. Magnani HN, Gallus A. Heparin-induced thrombocytopenia (HIT). A report of 1,478 clinical outcomes of patients treated with danaparoid (Orgaran) from 1982 to mid-2004. Thromb Haemost. 2006; 95:967–981. PMID: 16732376.
14. Al-Eidan FA. Pharmacotherapy of heparin-induced thrombocytopenia: therapeutic options and challenges in the clinical practices. J Vasc Nurs. 2015; 33:10–20. PMID: 25700733.

15. Alatri A, Armstrong AE, Greinacher A, et al. Results of a consensus meeting on the use of argatroban in patients with heparin-induced thrombocytopenia requiring antithrombotic therapy - a European Perspective. Thromb Res. 2012; 129:426–433. PMID: 22178575.

16. Tardy-Poncet B, Nguyen P, Thiranos JC, et al. Argatroban in the management of heparin-induced thrombocytopenia: a multicenter clinical trial. Crit Care. 2015; 19:396. PMID: 26556106.

17. Roca B, Roca M. The new oral anticoagulants: Reasonable alternatives to warfarin. Cleve Clin J Med. 2015; 82:847–854. PMID: 26651894.

18. van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate--a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010; 103:1116–1127. PMID: 20352166.
19. Coppens M, Eikelboom JW, Gustafsson D, Weitz JI, Hirsh J. Translational success stories: development of direct thrombin inhibitors. Circ Res. 2012; 111:920–929. PMID: 22982873.
20. Boehringer Ingelheim Pharmaceuticals, Inc. Boehringer Ingelheim Pradaxa product information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.;2015. Accesed January 10, 2016. at http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf.
21. Burness CB, McKeage K. Dabigatran etexilate: a review of its use for the prevention of venous thromboembolism after total hip or knee replacement surgery. Drugs. 2012; 72:963–986. PMID: 22564134.
22. Stangier J, Stähle H, Rathgen K, Roth W, Shakeri-Nejad K. Pharmacokinetics and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor, are not affected by moderate hepatic impairment. J Clin Pharmacol. 2008; 48:1411–1419. PMID: 18827075.

23. European Medicines Agency. EMA Pradaxa EPAR-product information. London, UK: European Medicines Agency;2015. Accesed January 10, 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000829/WC500041059.pdf.
24. Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet. 2008; 47:285–295. PMID: 18399711.
25. Hart RG, Eikelboom JW, Brimble KS, McMurtry MS, Ingram AJ. Stroke prevention in atrial fibrillation patients with chronic kidney disease. Can J Cardiol. 2013; 29(Suppl 7):S71–S78. PMID: 23790601.

26. Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015; 17:1467–1507. PMID: 26324838.

27. Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation--developed with the special contribution of the European Heart Rhythm Association. Europace. 2012; 14:1385–1413. PMID: 22923145.
28. Lippi G, Favaloro EJ. Recent guidelines and recommendations for laboratory assessment of the direct oral anticoagulants (DOACs): is there consensus? Clin Chem Lab Med. 2015; 53:185–197. PMID: 25241734.

29. Cuker A, Siegal D. Monitoring and reversal of direct oral anticoagulants. Hematology Am Soc Hematol Educ Program. 2015; 2015:117–124. PMID: 26637710.

30. Canadian Agency for Drugs and Technologies in Health. Anticoagulation monitoring and reversal strategies for Dabigatran, Rivaroxaban, and Apixaban: A review of clinical effectiveness. Ottawa, Canada: Canadian Agency for Drugs and Technologies in Health;2012. Accesed January 10, 2016. https://www.cadth.ca/media/pdf/TR0002_New_Oral_Anticoagulants.pdf.
31. Cuker A. Laboratory measurement of the non-vitamin K antagonist oral anticoagulants: selecting the optimal assay based on drug, assay availability, and clinical indication. J Thromb Thrombolysis. 2016; 41:241–247. PMID: 26386967.

32. Lippi G, Ardissino D, Quintavalla R, Cervellin G. Urgent monitoring of direct oral anticoagulants in patients with atrial fibrillation: a tentative approach based on routine laboratory tests. J Thromb Thrombolysis. 2014; 38:269–274. PMID: 24811247.

33. Baglin T, Keeling D, Kitchen S. British Committee for Standards in Haematology. Effects on routine coagulation screens and assessment of anticoagulant intensity in patients taking oral dabigatran or rivaroxaban: guidance from the British Committee for Standards in Haematology. Br J Haematol. 2012; 159:427–429. PMID: 22970737.

34. Chin PK, Patterson DM, Zhang M, et al. Coagulation assays and plasma fibrinogen concentrations in real-world patients with atrial fibrillation treated with dabigatran. Br J Clin Pharmacol. 2014; 78:630–638. PMID: 24592919.

35. Douxfils J, Lessire S, Dincq AS, et al. Estimation of dabigatran plasma concentrations in the perioperative setting. An ex vivo study using dedicated coagulation assays. Thromb Haemost. 2015; 113:862–869. PMID: 25519251.
36. Greinacher A, Thiele T, Selleng K. Reversal of anticoagulants: an overview of current developments. Thromb Haemost. 2015; 113:931–942. PMID: 25832311.

37. Eikelboom JW, Quinlan DJ, van Ryn J, Weitz JI. Idarucizumab: The antidote for reversal of dabigatran. Circulation. 2015; 132:2412–2422. PMID: 26700008.
38. Glund S, Stangier J, Schmohl M, et al. Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo-controlled, double-blind phase 1 trial. Lancet. 2015; 386:680–690. PMID: 26088268.

39. Glund S, Moschetti V, Norris S, et al. A randomised study in healthy volunteers to investigate the safety, tolerability and pharmacokinetics of idarucizumab, a specific antidote to dabigatran. Thromb Haemost. 2015; 113:943–951. PMID: 25789661.

40. Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015; 373:511–520. PMID: 26095746.

41. Glund S, Stangier J, van Ryn J, et al. Restarting dabigatran etexilate 24 h after reversal with idarucizumab and redosing idarucizumab in healthy volunteers. J Am Coll Cardiol. 2016; 67:1654–1656. PMID: 27150693.

42. Hu TY, Vaidya VR, Asirvatham SJ. Reversing anticoagulant effects of novel oral anticoagulants: role of ciraparantag, andexanet alfa, and idarucizumab. Vasc Health Risk Manag. 2016; 12:35–44. PMID: 26937198.
43. Grottke O, van Ryn J, Spronk HM, Rossaint R. Prothrombin complex concentrates and a specific antidote to dabigatran are effective ex-vivo in reversing the effects of dabigatran in an anticoagulation/liver trauma experimental model. Crit Care. 2014; 18:R27. PMID: 24499559.

44. Grottke O, Aisenberg J, Bernstein R, et al. Efficacy of prothrombin complex concentrates for the emergency reversal of dabigatran-induced anticoagulation. Crit Care. 2016; 20:115. PMID: 27125504.

45. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361:1139–1151. PMID: 19717844.

46. Connolly SJ, Ezekowitz MD, Yusuf S, Reilly PA, Wallentin L. Randomized Evaluation of Long-Term Anticoagulation Therapy Investigators. Newly identified events in the RE-LY trial. N Engl J Med. 2010; 363:1875–1876. PMID: 21047252.

47. Connolly SJ, Wallentin L, Yusuf S. Additional events in the RE-LY trial. N Engl J Med. 2014; 371:1464–1465. PMID: 25251519.

48. Nagarakanti R, Wallentin L, Noack H, et al. Comparison of characteristics and outcomes of dabigatran versus warfarin in hypertensive patients with atrial fibrillation (from the RE-LY Trial). Am J Cardiol. 2015; 116:1204–1209. PMID: 26282726.

49. Brambatti M, Darius H, Oldgren J, et al. Comparison of dabigatran versus warfarin in diabetic patients with atrial fibrillation: Results from the RE-LY trial. Int J Cardiol. 2015; 196:127–131. PMID: 26093161.

50. Böhm M, Ezekowitz MD, Connolly SJ, et al. Changes in renal function in patients with atrial fibrillation: An analysis from the RE-LY trial. J Am Coll Cardiol. 2015; 65:2481–2493. PMID: 26065986.
51. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009; 361:2342–2352. PMID: 19966341.

52. Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014; 129:764–772. PMID: 24344086.

53. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013; 368:709–718. PMID: 23425163.

54. Mirdamadi A. Dabigatran, a direct thrombin inhibitor, can be a life-saving treatment in heparin-induced thrombocytopenia. ARYA Atheroscler. 2013; 9:112–114. PMID: 23690810.
55. Anniccherico FJ, Alonso JL, Urbieta M, Pérez Ricarte S. Dabigatran as a therapeutic possibility in heparin-induced thrombocytopenia type II. An Sist Sanit Navar. 2012; 35:521–524. PMID: 23296239.
56. Fieland D, Taylor M. Dabigatran use in a postoperative coronary artery bypass surgery patient with nonvalvular atrial fibrillation and heparin-PF4 antibodies. Ann Pharmacother. 2012; 46:e3. PMID: 22202498.

57. Bircan HA, Alanoglu EG. Massive pulmonary embolism in a patient with heparin induced thrombocytopenia: successful treatment with dabigatran. Eurasian J Med. 2016; 48:65–68. PMID: 27026768.

58. Walenga JM, Prechel M, Jeske WP, et al. Rivaroxaban--an oral, direct Factor Xa inhibitor--has potential for the management of patients with heparin-induced thrombocytopenia. Br J Haemotol. 2008; 143:92–99.
59. Ng HJ, Than H, Teo EC. First experiences with the use of rivaroxaban in the treatment of heparin-induced thrombocytopenia. Thromb Res. 2015; 135:205–207. PMID: 24974053.

60. Hantson P, Lambert C, Hermans C. Rivaroxaban for arterial thrombosis related to heparin-induced thrombocytopenia. Blood Coagul Fibrinolysis. 2015; 26:205–206. PMID: 25255239.

61. Sartori M, Favaretto E, Cini M, Legnani C, Cosmi B. Rivaroxaban in the treatment of heparin-induced thrombocytopenia. J Thromb Thrombolysis. 2015; 40:392–394. PMID: 25804370.

62. Abouchakra L, Khabbaz Z, Abouassi S, Badaoui G. Rivaroxaban for treatment of heparin-induced thrombocytopenia after cardiac surgery: A case report. J Thorac Cardiovasc Surg. 2015; 150:e19–e20. PMID: 26055438.

63. Tardy-Poncet B, Piot M, Montmartin A, Burdier A, Chalayer E, Tardy B. Delayed-onset heparin-induced thrombocytopenia without thrombosis in a patient receiving postoperative thromboprophylaxis with rivaroxaban. Thromb Haemost. 2015; 114:652–654. PMID: 26062524.

64. Linkins LA, Warkentin TE, Pai M, et al. Design of the rivaroxaban for heparin-induced thrombocytopenia study. J Thromb Thrombolysis. 2014; 38:485–492. PMID: 24549975.

65. Linkins LA, Warkentin TE, Pai M, et al. Rivaroxaban for treatment of suspected or confirmed heparin-induced thrombocytopenia study. J Thromb Haemost. 2016; 14:1206–1210. PMID: 27061271.

66. Walenga JM, Prechel M, Hoppensteadt D, et al. Apixaban as an alternate oral anticoagulant for the management of patients with heparin-induced thrombocytopenia. Clin Appl Thromb Hemost. 2013; 19:482–487. PMID: 23780399.

67. Larsen PB, Jørgensen M, Friis-Hansen L, Ingeberg S. Apixaban used for the management of heparin-induced thrombocytopenia in a 72-year-old woman with lung cancer. Clin Case Rep. 2015; 3:987–989. PMID: 26732728.

68. Sharifi M, Bay C, Vajo Z, Freeman W, Sharifi M, Schwartz F. New oral anticoagulants in the treatment of heparin-induced thrombocytopenia. Thromb Res. 2015; 135:607–609. PMID: 25613925.
69. Krauel K, Hackbarth C, Fürll B, Greinacher A. Heparin-induced thrombocytopenia: in vitro studies on the interaction of dabigatran, rivaroxaban, and low-sulfated heparin, with platelet factor 4 and anti-PF4/heparin antibodies. Blood. 2012; 119:1248–1255. PMID: 22049520.

70. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011; 365:883–891. PMID: 21830957.

71. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365:981–992. PMID: 21870978.
72. Baker WL, Phung OJ. Systematic review and adjusted indirect comparison meta-analysis of oral anticoagulants in atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012; 5:711–719. PMID: 22912382.

73. Lip GY, Larsen TB, Skjøth F, Rasmussen LH. Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2012; 60:738–746. PMID: 22575324.

Fig. 1
Pathophysiology of Heparin-Induced Thrombocytopenia (HIT) and new oral anticoagulants. Heparin-induced thrombocytopenia is an immune-mediated complication of exposure to unfractionated or low-molecular-weight heparin. Platelet factor 4 (PF4) released from α-granule of platelet binds to polyanions such as bacteria and heparin, and exposes previously masked epitope, which leads to formation of anti-PF4-IgG antibodies. These antibodies have ability to bind PF4-heparin complexes, and the PF4-heaprin-IgG immune complex activates platelets through binding to FcγRIIa of the platelets, which in turn activates and aggregates platelets. The release of additional PF4 from activated platelets and thrombin activation lead to increased consumption of platelets and eventually thrombocytopenia. The immune complexes also activate monocytes through binding to FcγRI, which can stimulate production of tissue factors from endothelial cells. Dabigatran, a univalent direct thrombin inhibitor, and the FXa inhibitors such as rivaroxaban and apixaban, could serve as an alternative anticoagulants for HIT and thrombosis prophylaxis. Abbreviations: PF4, platelet factor 4; TF, tissue factor; IIa, thrombin; Va, activated factor V; Xa, activated factor X; VIIIa, activated factor VIII; IXa, activated factor IX; AT, antithrombin.

Table 2
Case studies investigating the use of NOACs to treat heparin-induced thrombocytopenia.
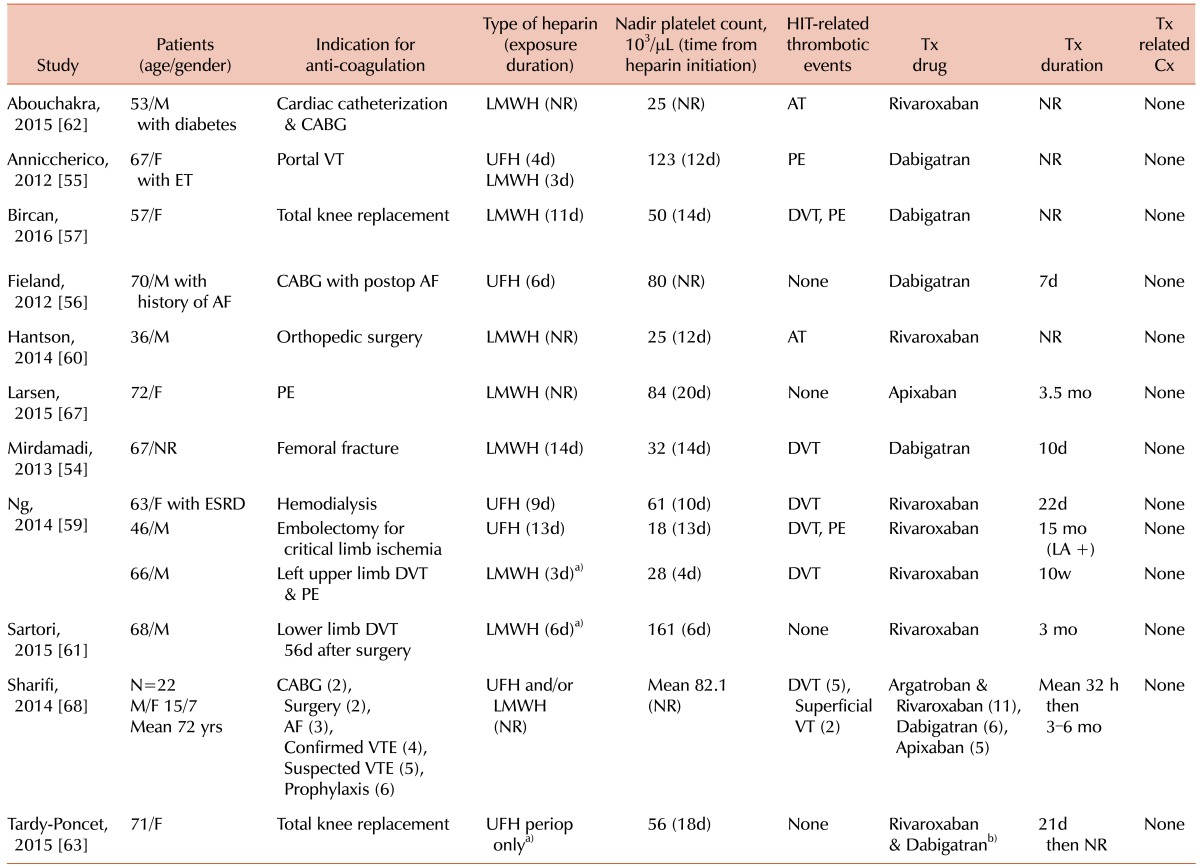
a)History of prior exposure. b)Due to persistent thrombocytopenia.
Abbreviatrions: Tx, Treatment; Cx, complications; NR, not reported; AT, Arterial thrombosis; DVT, deep vein thrombosis; VT, venous thrombosis; PE, pulmonary embolism; UFH, unfractionated heparin; LMWH, low-molecular-weight heparin; VTE, venous thromboembolism; HIT, heparin-induced thrombocytopenia; ET, essential thrombocytopenia; ESRD, end stage renal failure; LA, lupus anticoagulant; AF, atrial fibrillation; CABG, coronary artery bypass graft.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download