TO THE EDITOR: The International Randomized Interferon versus STI-571 study was the first randomized trial to demonstrate the prognostic significance of monitoring BCR-ABL1 transcripts by real-time quantitative polymerase chain reaction (RT-PCR) [1], and defined a major molecular response (MMR) after treatment as a 3-log10 reduction from the baseline value in the level of measured BCR-ABL1 transcripts [1]. On the other hand, an international scale (IS) was proposed as an absolute value of 0.1% BCR-ABL1, which corresponds to a 3-log10 reduction from the baseline value, for MMR, in an effort made by international experts to standardize BCR-ABL1 quantification [23].
According to European Leukemia Net guidelines, optimal response to first-line imatinib therapy was defined as complete hematologic response and at least minor cytogenetic response at 3 months, at least partial cytogenetic response at 6 months, complete cytogenetic response at 12 months, and MMR at 18 months [4]. Recently, several investigators suggested that early molecular responses (EMRs) within the first 6 months of therapy have prognostic significance [56].
The molecular response of CML patients to tyrosine kinase inhibitor (TKI) treatment can be evaluated by BCR-ABL1 mRNA levels on the IS or by log reduction from the baseline level considered by the laboratory. This study was performed to determine whether there is any difference between an IS-based RT-PCR kit and a non-IS-based RT-PCR kit for EMR determination.
Bone marrow aspirates at diagnosis and 3 months after start of TKI therapy were collected from 19 patients diagnosed with chronic phase or accelerated phase CML at Ajou University Hospital between June 2008 and September 2011.When possible, samples were processed within 24 hours of collection. Samples that could not be immediately processed were stored at -70℃ prior to processing. We reviewed the patients' medical records, including age at diagnosis, gender, disease phase, and medication. This study was performed under approval of the institutional review board of Ajou University Hospital.
We extracted RNA from mononuclear cells isolated from bone marrow by the guanidinium isothiocyanate-phenol chloroform method as described in a previous study [7]. RT-PCR was performed using the LightCycler t(9;22) quantification kit (Roche Molecular Systems, Inc., Branchburg, NJ, USA) and the BCR-ABL Mbcr IS-MMR Dx kit (IPSOGEN, Marseille, France) according to the manufacturers' instructions. The former was designed to detect b3a2, b2a2 and e1a2 fusion transcripts and the latter is a kit for the quantification of b3a2 and b2a2 fusion gene transcripts. The BCR-ABL Mbcr IS-MMR Dx kit has been designed according to the Europe Against Cancer studies [89], is compliant with updated international recommendations, and contains an IS-MMR calibrator aligned with the IS, allowing conversion of normalized copy number (NCN) to the IS in contrast to the LightCycler t(9;22) quantification kit, which does not provide IS values.
The RT-PCR was performed using LightCycler 2.0 (Roche Diagnostics Ltd, Rotkreuz, Switzerland) for both kits. For the LightCycler t(9;22) quantification kit, expression levels of BCR-ABL1 mRNA were analyzed relative to those of G6PDH. Log reductions in BCR-ABL1 levels from baseline were calculated with the LightCycler kit using the baseline value of 0.06, which is the median level of bone marrow samples from 20 newly diagnosed CML patients in our laboratory. We considered ≥1 log10 reduction at 3 months as EMR. For the BCR-ABL Mbcr IS-MMR Dx kit, standard curves obtained on plasmid standard dilutions were used to calculate copy number (CN) and NCN was derived from the equation (CN of BCR-ABL1/CN of ABL)×100. The NCN value was converted to the IS value using the equation (NCN of sample×assigned value of the IS-MMR-Calibrator/NCN of the calibrator). Nine quality criteria were used to validate the RT-PCR results as recommended by the manufacturer. We considered ≤10% IS at 3 months as EMR.
Data analysis was performed using Microsoft Excel 2007 (Microsoft, Redmond, Washington) and MedCalc (MedCalc Software, Ostend, Belgium). To compare EMR determination by the two methods, the McNemar test was used and a P value less than 0.05 was considered statistically significant. Linear regression was used for correlation analysis of the BCR-ABL1 quantification kits.
The mean age of the patients was 44±15 years, and male to female ratio was 1.4:1. Fourteen patients were in chronic phase, and 5 in accelerated phase. Imatinib mesylate at 400 mg was administered daily to all patients except for one, to whom 600mg was administered. The mean time interval for follow-up of quantification of BCR-ABL1 transcripts was 3.1(2.0–4.5) months.
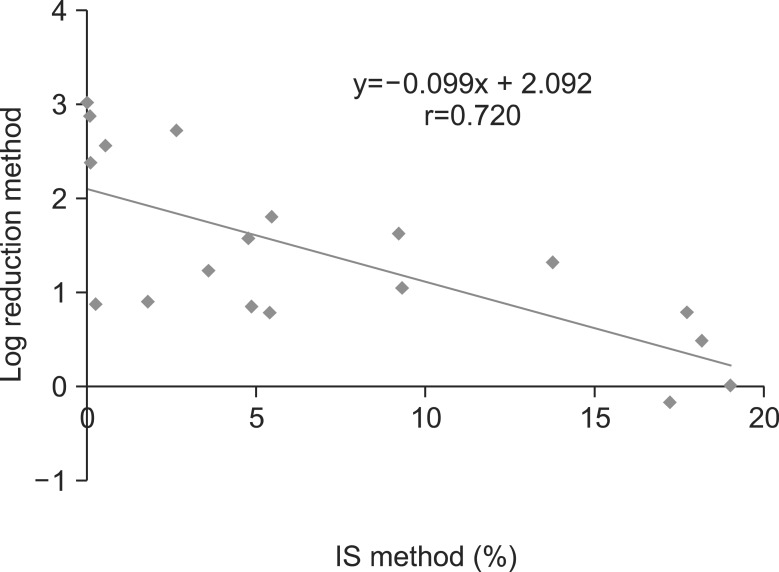
The mean BCR-ABL1 transcript levels as determined by the LightCycler kit and the IPSOGEN kit for initial samples were 0.057±0.086 (median, 0.03) and 60.6±17.8% (median, 63.0), respectively. The mean BCR-ABL1 transcript levels as determined by the LightCycler kit and the IPSOGEN kit of follow-up samples were 0.012±0.023 (median, 0.004) and 7.0±6.9% (median, 4.9), respectively. The correlation coefficient (r) between the two methods was 0.720, showing a moderately linear correlation (Fig. 1).
The IS method and the log reduction method showed a concordance rate of 74% (14/19) for the determination of EMR status. Four of 5 patients with discrepancies were determined to achieve EMR by the IS method but not by the log reduction method, and 1 vice versa. The P value with the McNemar test was 0.3750, indicating that there was no statistically significant difference between the two methods in determining EMR. Sixteen of 17 patients for whom the survival data were available are alive, except for one whose BCR-ABL1 transcript level was determined as no EMR by both methods. Clinical and laboratory findings of the patients showing a discrepancy between the log reduction method and the IS method are summarized in Table 1.
European LeukemiaNet guidelines recommend measuring BCR-ABL1 transcript levels during treatment for every 3 months until achievement of MMR and every 6 months thereafter [4]. Accumulation of the serial quantification data for BCR-ABL1 transcript levels during TKI therapy introduced a new era in monitoring therapy response in CML. Some investigators found that the depth of molecular response at 3 months could identify a subset of patients with relatively poor survival and prognosis [561011]. A new EMR definition was suggested as the 3-month BCR-ABL1 transcript level ≤10% IS or ≥1 log reduction [5611] and was used in the present study.
Accurate measurement of baseline or normalized values of BCR-ABL1 fusion transcripts is a prerequisite for monitoring therapeutic efficacy over time and identifying patients who are unresponsive to therapy in the early phase of treatment [12]. Given the current lack of agreement between different measurement methods, it is necessary to know the extent to which methods disagree and whether the differences will cause problems in clinical interpretation [2]. In a study with 248 newly diagnosed CML-CP patients who underwent imatinib therapy, the investigators concluded that the IS method better differentiates the patients according to their predictive values to progression-free survival by EMR as well as by MMR than the log reduction method [11]. A significantly higher number of patients were considered as having an EMR by the IS method than by the log reduction method. Our data also showed a higher number of patients as having an EMR by the IS method than by the log reduction method, but this result is statistically insignificant. The distribution of patients with BCR-ABL1 transcript levels in the present study is akin to previous studies [611].
RT-PCR data for BCR-ABL1 transcript levels may vary by approximately 0.5 to 1 log depending on the laboratory conducting analysis [13]. Many factors influence the assay, such as inter-sample variation in interfering substances, sample processing within 24 hours after blood collection, efficiency of reverse transcription, inter-assay variation in quality or quantity of PCR reagents, and thermal cycler performance [14]. It seems important for molecular laboratories using non-IS-based kits to establish their own quality criteria, adhere to it over the entire procedure, and make every effort in maintaining quality of the assay. Although the use of IS might be a breakthrough, it would be cumbersome and labor-intensive for some laboratories to derive the laboratory-specific conversion factors (CFs) that enable locally derived results to be converted to the IS. In addition, there are a number of obvious issues with CFs, for example, lack of full information through the analyses in the 50% of laboratories who fail to achieve the defined performance criteria [215].
Recently, an increasing number of manufacturers have produced IS-based RT-PCR kits for the major BCR-ABL1 transcript level that do not require laboratory-specific CFs. The BCR-ABL Mbcr IS-MMR Dx kit used in this study is one of them and adopts 9 quality criteria, including Ct value replicate variations and slope and r2 for standard curves, to validate the RT-PCR results. Theoretically, initial samples of the CML patients should measure 100% IS, but in this study, they measured 61% IS with a coefficient of variation of 29%, which is considered allowable.
Although the present study has limitations such as a small number of cases and lack of assessment of sensitivity between methods, our data failed to show a significant difference in determination of EMR status of CML patients receiving TKI therapy by the IS or log reduction method. A multi-institutional study with enough cases to validate the usefulness of the log reduction method in monitoring EMR status is required.
Notes
References
1. Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003; 349:1423–1432. PMID: 14534335.

2. Branford S, Fletcher L, Cross NC, et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood. 2008; 112:3330–3338. PMID: 18684859.

3. Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006; 108:28–37. PMID: 16522812.

4. Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009; 27:6041–6051. PMID: 19884523.

5. Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012; 30:232–238. PMID: 22067393.
6. Hanfstein B, Müller MC, Hehlmann R, et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML). Leukemia. 2012; 26:2096–2102. PMID: 22446502.

7. Park JS, Yi JW, Jeong SH, et al. Comparison of multiplex reverse transcription polymerase chain reaction and conventional cytogenetics as a diagnostic strategy for acute leukemia. Int J Lab Hematol. 2008; 30:513–518. PMID: 18983303.

8. Gabert J, Beillard E, van der Velden VH, et al. Standardization and quality control studies of 'real-time' quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia - a Europe Against Cancer program. Leukemia. 2003; 17:2318–2357. PMID: 14562125.

9. Beillard E, Pallisgaard N, van der, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using 'real-time' quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) - a Europe against cancer program. Leukemia. 2003; 17:2474–2486. PMID: 14562124.

10. Quintás-Cardama A, Kantarjian H, Jones D, et al. Delayed achievement of cytogenetic and molecular response is associated with increased risk of progression among patients with chronic myeloid leukemia in early chronic phase receiving high-dose or standard-dose imatinib therapy. Blood. 2009; 113:6315–6321. PMID: 19369233.

11. Qin YZ, Jiang Q, Jiang H, et al. Which method better evaluates the molecular response in newly diagnosed chronic phase chronic myeloid leukemia patients with imatinib treatment, BCR-ABL(IS) or log reduction from the baseline level? Leuk Res. 2013; 37:1035–1040. PMID: 23810191.

12. Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006; 355:2408–2417. PMID: 17151364.
13. Fava C, Kantarjian H, Cortes J. Molecular resistance: an early indicator for treatment change? Clin Lymphoma Myeloma Leuk. 2012; 12:79–87. PMID: 22285607.

14. Stanoszek LM, Crawford EL, Blomquist TM, Warns JA, Willey PF, Willey JC. Quality control methods for optimal BCR-ABL1 clinical testing in human whole blood samples. J Mol Diagn. 2013; 15:391–400. PMID: 23541592.

15. Cross NC, White HE, Müller MC, Saglio G, Hochhaus A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia. 2012; 26:2172–2175. PMID: 22504141.

Fig. 1
Correlation between the international scale (IS) values and the log10 reductions in BCR-ABL1 levels in the chronic myeloid leukemia patients at 3 months after imatinib mesylate therapy. Abscissa indicates IS values obtained by the BCR-ABL Mbcr IS-MMR Dx kit. Ordinate indicates the log10 reductions in BCR-ABL1 levels from baseline obtained by the LightCycler t(9;22) quantification kit.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download