INTRODUCTION
Hemophilia A is one of the most common F8 gene mutation-induced bleeding disorders. Patients with hemophilia lack coagulation factors. Accordingly, replacement therapy with concentrates of the insufficient factors has been a mainstay since the late 1960s. Factor replacement therapy can improve both the quality of life and life expectancy of patients with hemophilia. However, many patients experience factor concentrate-related complications. One major complication is the development of inhibitors to factor concentrates. Inhibitors that are immunoglobulin G (IgG) antibodies [1] bind to the active sites of factor VIII (FVIII) or factor IX (FIX) molecules and neutralize the clotting functions. The development of an inhibitor renders hemostasis more difficult, increases disability [2], and reduces the quality of life [3] and life expectancy [4].
The incidence of inhibitor development is known to be approximately 30% among patients with severe hemophilia A [5], 0.9–7% among those with mild to moderate hemophilia A [6], and 1.5–3% among those with severe hemophilia B [7]. The reported prevalence of inhibitors is as high as 8.9–16.4% [8] in patients with severe hemophilia A, although this varies widely by study population.
Inhibitors usually develop during an early stage of treatment; 95% of inhibitors occur during the first 50 exposure days (EDs), and >99% during the first 100 EDs [9]. Several risk factors are known to influence inhibitor development including gene mutations, major histocompatibility complex expression, intensity of therapy, family history of inhibitors, and ethnicity.
Type of inhibitors can be classified as transient or persistent. Large-dose factor concentrates or bypassing agents can be used against inhibitors. However the ultimate treatment goal for patients with inhibitor is the eradication of inhibitors. Although ITI has been widely known to reduce inhibitor by 79–87% [10], implementation of ITI is very tricky in terms of eligibility of patient, timing of ITI start and the dosage of factor concentrates. It is of note that some inhibitors may disappear spontaneously without ITI which demands huge amount of factor concentrates. Among patients with maximum inhibitor titers >10 BU/mL, 3.4% exhibited spontaneous inhibitor disappearance [11].
We investigated the long-term course of inhibitor development to factor VIII concentrates at a single center. Through this study, we hoped to elucidate the characteristics of inhibitors and aimed to find the proper indications and timing for ITI.
Go to : 
MATERIALS AND METHODS
Study population
The medical records of hemophilia A patients registered in the Korea Hemophilia Foundation (KHF) from 1991 to 2014 were investigated retrospectively. In 1991, the Ministry of Health and Welfare commissioned KHF with the registration of Korean patients with hemophilia. Since then, patient data have been systemically filed in the KHF. Patient data were collected from the medical records and electronic database of the KHF. The collected data included phenotypic severity, age at diagnosis of hemophilia A, exposure days, interval of inhibitor testing, age at inhibitor detection, peak inhibitor titer, duration of inhibitor presence, treatment strategy, and type of F8 gene mutation.
Inhibitor testing
Inhibitor titers were measured using the Bethesda assay. One Bethesda Unit (BU) is defined as the amount of an inhibitor that will neutralize 50% of 1 unit of FVIII:C in normal plasma after a 120-minute incubation at 37℃. The cut-off value of inhibitor positivity in the Bethesda assay was 0.6 BU/mL. The KHF recommends an inhibitor testing frequency of every 6 months until 20 years of age, and once every 12 months thereafter. This recommendation was modified from that of the United Kingdom Haemophilia Centre Doctors Organization's (UKHCDO) [12] and other reports [13]. Although hemophilia therapy was commenced in Korea in the mid-1970s, the performance of regular inhibitor evaluations began after the establishment of the KHF in 1991.
Classification of patient groups
We classified inhibitors according to persistency as persistent or transient inhibitors. Persistent inhibitor was defined as an inhibitor that had consistently been present since the initial factor VIII concentrate challenge. Transient inhibitor was defined as an inhibitor that disappeared within a short period of time while the patient remained on standard treatment [8].
Patients were differentiated into 4 groups according to the historical peak titer: low, moderate, high, and very high titer. The low, moderate, high, and very high titer groups included patients with recorded inhibitor levels of 0.6 to <2 BU/mL, 2 to <5 BU/mL, 5 to <10 BU/mL, and ≥10 BU/mL, respectively. Regarding the cut-off value for a high inhibitor titer, some researchers have used 10 BU/mL, whereas others have used 5 BU/mL. In addition to those 2 values, we used 2 BU/mL to define the moderate titer group. This was selected because some researchers reported that most of the observed transient inhibitor titers were <2 BU/mL [1415]. In addition, 2 BU/mL was considered to indicate clinical tolerance achieved by low-dose ITI [16].
Treatment strategy
For bleeding events in patients with inhibitors, an injection of large-dose (more than 50 U/kg per dose) factor VIII concentrate and bypassing agent (BPA) infusion therapy was considered. Prothrombin complex concentrate (PCC) was used prior to the launch of activated PCC (aPCC) or recombinant activated factor VII (rFVIIa) in Korea. In addition to large-dose therapy and BPA, the usual hemostatic dose (20–25 U/kg) was also used for patients with inhibitors. We investigated treatment strategies such as on-demand therapy or prophylaxis. In particular, low-dose ITI comprising thriceweekly prophylaxis at 25 U/kg was expected to eliminate inhibitors. Before 2008, ITI practice was not officially allowed. To implement low-dose ITI (25 U/kg thrice weekly) without approval from an authority, clinicians reduced the 50-U/kg dosage allowed for use as on-demand therapy to 25 U/kg and infused this dosage thrice weekly.
Statistical analysis
For statistical analysis and comparison between patients with persistent and transient inhibitors, we subjected each variable to the Mann–Whitney and chi-square tests. To compare the durations of inhibitor persistence in low, moderate, high, and very high titer groups of patients with transient inhibitors, the Kruskal–Wallis test and Bonferroni correction post-hoc test were utilized. We also conducted a Kaplan–Meier survival analysis to determine the persistence of transient inhibitors. Onset of survival analysis was the time of inhibitor detection and event was the time of disappearance of inhibitors. The data of the patients who had died during the study period and couldn't be followed up was censored. The chi-square test and Fisher's exact test were used to assess the influence of F8 gene mutations on inhibitor risk.
Go to : 
RESULTS
Patient characteristics
In total, 1,634 hemophilia A patients were investigated in this study. Among them, 1,154 (70.6%), 290 (17.8%), and 184 (11.3%) patients had severe, moderate, and mild disease, respectively. The remaining 6 patients' phenotypes were unknown.
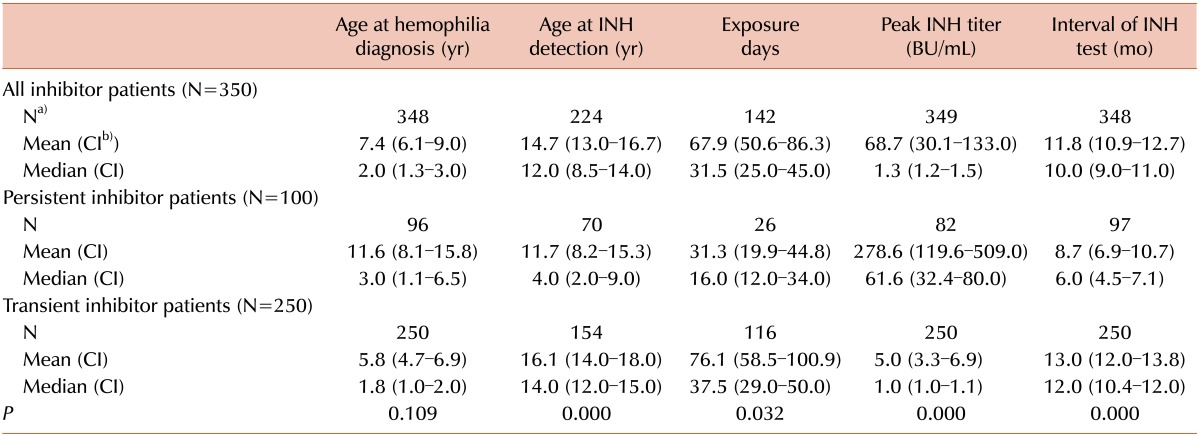
The mean ages at hemophilia diagnosis of all inhibitor patients, persistent inhibitor patients, and transient inhibitor patients were 7.4 years, 11.6 years, and 5.8 years, respectively (Table 1). The difference in the age at hemophilia diagnosis between persistent and transient inhibitor patients was not statistically significant (P=0.109). However, persistent inhibitors were detected significantly later than transient inhibitors (P<0.001).
In 1991, when registration initially began, 591 hemophilia A patients with a median age of 15.0 years were registered. During the same year, 153 (25.9%) patients were recorded as inhibitor-positive and had a median age of 13.5 years. The proportion of newly developed inhibitor patients in the first 5 years of registration was relatively high. It is because already-existed inhibitor patients diagnosed elsewhere were registered during the early period. The annual number of new registrants gradually decreased to 40 in 2014, although the proportion of patients with novel inhibitor development varied (Fig. 1). It is of note that the proportion of newly diagnosed inhibitor patients suddenly increased in 2005 and 2010 when 1st and 3rd generation full-length recombinant FVIII concentrates have launched in Korea.
Inhibitor characteristics
Among the 1,634 studied hemophilia A patients, 350 (21.4%) tested positive via the Bethesda assay at least once during the study period. Of these, 100 (28.6%) patients were classified as having persistent inhibitors. From these 100 patients, 32 gained immune tolerance after ITI, which comprised 16 intermediate doses and 16 low doses of ITI. The remaining 250 patients' inhibitors were later found to be transient (15.3% of total hemophilia A patients, 71.4% of patients with inhibitors). The median intervals of inhibitor testing in the persistent and transient inhibitor patients were 6.0 and 12.0 months, respectively (P<0.001). The EDs of factor VIII concentrate before inhibitor development could be investigated in only 142 inhibitor patients because many patients with hemophilia had been referred to KHF after receiving multiple factor concentrate injections (patients could not recall the numbers) or even after inhibitor detection. The median EDs of persistent and transient inhibitor patients were 16.0 and 37.5 EDs (P=0.032). The median peak titers of persistent and transient inhibitors were 61.6 BU/mL and 1.0 BU/mL, respectively (Table 1, P<0.001)
Out of 1,154 severe patients, 318 (27.6%) developed inhibitors. Among them, 224 (70.4%) were regarded to have transient inhibitors. Among 290 moderate patients, 29 (10.0%) were recorded as inhibitor-positive at least once.Twenty-three (79.3%) patients were classified as having transient inhibitor. Among 184 mild phenotype patients, only 3 (1.6%) developed inhibitors, and all cases were transient. The severer phenotypes patients have, the more frequently patients developed inhibitors (P<0.001). However the proportion of persistent inhibitors to transient inhibitors was not significantly higher in severe patients in comparison with mild and moderate patients (P=0.221) (Fig. 2).
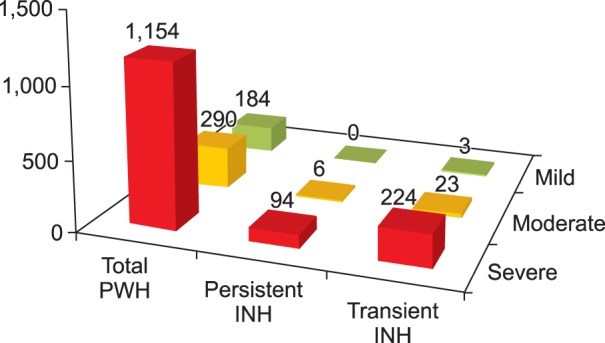 | Fig. 2Inhibitor status according to hemophilia A phenotypes. The severer phenotypes patients have, the more frequent inhibitors the patients developed (P<0.001). However the proportion of persistent inhibitors to transient inhibitors was not significantly higher in severe patients in comparison with the mild and moderate patients (P=0.221). Abbreviations: PWH, patient with hemophilia; INH, inhibitor. |
Persistence of inhibitors
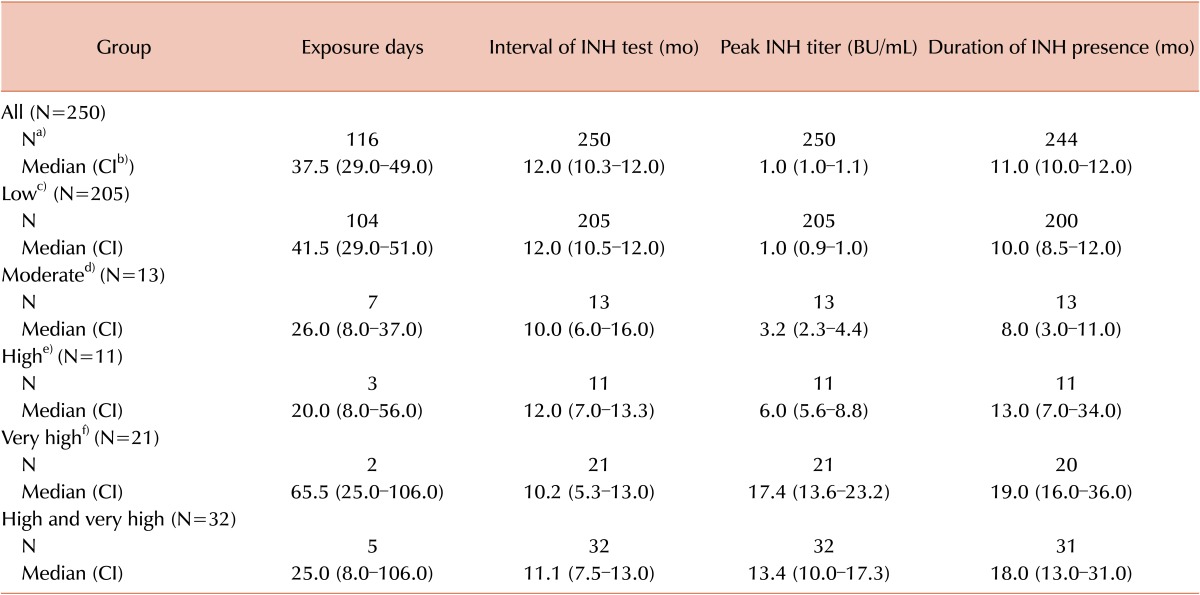
The median peak titer of transient inhibitors was 1.0 BU/mL, and transient inhibitors were present for 11.0 months. In the low, moderate, high, and very high titer groups, the median durations of inhibitor persistence were 10.0, 8.0, 13.0, and 19.0 months, respectively (Table 2). This difference in duration among the 4 groups was found to be statistically significant (Kruskal–Wallis test, P=0.001). After a post-hoc test using the Bonferroni correction, significant differences in duration were observed between the low and very high titer groups (P<0.001) and moderate and very high titer groups (P=0.004). This finding indicates that inhibitors at levels ≥10 BU/mL persist for longer than those with levels <5 BU/mL. A Kaplan–Meier survival analysis yielded the same results (log-rank, P=0.001; Fig. 3).
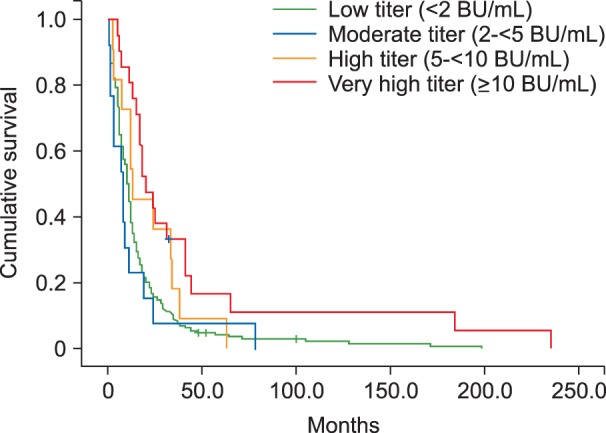 | Fig. 3Kaplan–Meier survival curve of transient inhibitors according to peak titer. The duration of inhibitor presence was longer in very high titer, transient inhibitor group than in other 3 groups (P=0.001). |
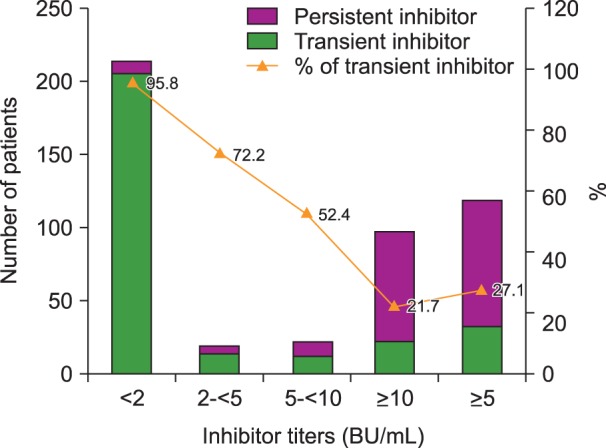
Among all patients with inhibitors (transient and persistent), a total of 214 had a low titer. Among these patients, 205 (95.8%) eventually became inhibitor negative. Patients with transient inhibitors comprised 13 of 18 (72.2%), 11 of 21 (52.4%), and 21 of 97 (21.7%) patients in the moderate, high, and very high titer groups, respectively. Among patients with inhibitor titers >5 BU/mL, 32 of 118 (27.1%) had transient inhibitors (Fig. 4).
Regarding inhibitor recurrence, a total of 155 episodes of inhibitor persistency were recorded for 71 of 250 patients with transient inhibitors (28.4%). A median of 2.0 (range, 2.0–4.0) inhibitor recurrences were recorded per patient. The median time interval to inhibitor reappearance was 3.0 years. The mean inhibitor titers of the first (N=71) and second (N=71) episodes were 4.1 BU/mL (median, 1.1, confidence interval [CI]: 1.0–1.2) and 1.3 BU/mL (median, 0.9, CI: 0.8–1.0; P=0.008). The median inhibitor titer of the third (N=12) episode was 0.7 BU/mL (CI: 0.6–0.7). Only 1 patient experienced a fourth recurrent episode. Inhibitors resulting from recurrent episodes disappeared within 8.0 months without ITI (Table 3).
Thirty-three of 250 (13.2%) patients with transient inhibitors were recorded as inhibitor-positive for >24 months (median, 35 mo). The median interval of inhibitor testing was 12 months, and the median historical peak titer was 1.2 BU/mL.
Treatment strategy
Among the 205 patients with low-titer transient inhibitors, only 12 had been treated with large-dose factor replacement or BPA as on-demand therapy. The remaining 238 patients were treated with doses of 20–25 U/kg. Low-dose ITI achieved immune tolerance in 16 patients with persistent inhibitors including 8 (10.4%) of 77 with very high persistent inhibitor titers, 4 (40%) of 10 with high persistent inhibitor titers, 2 (40%) of 5 with moderate persistent inhibitor titers, and 2 (25%) of 8 with low persistent inhibitor titers.
Mutation analysis
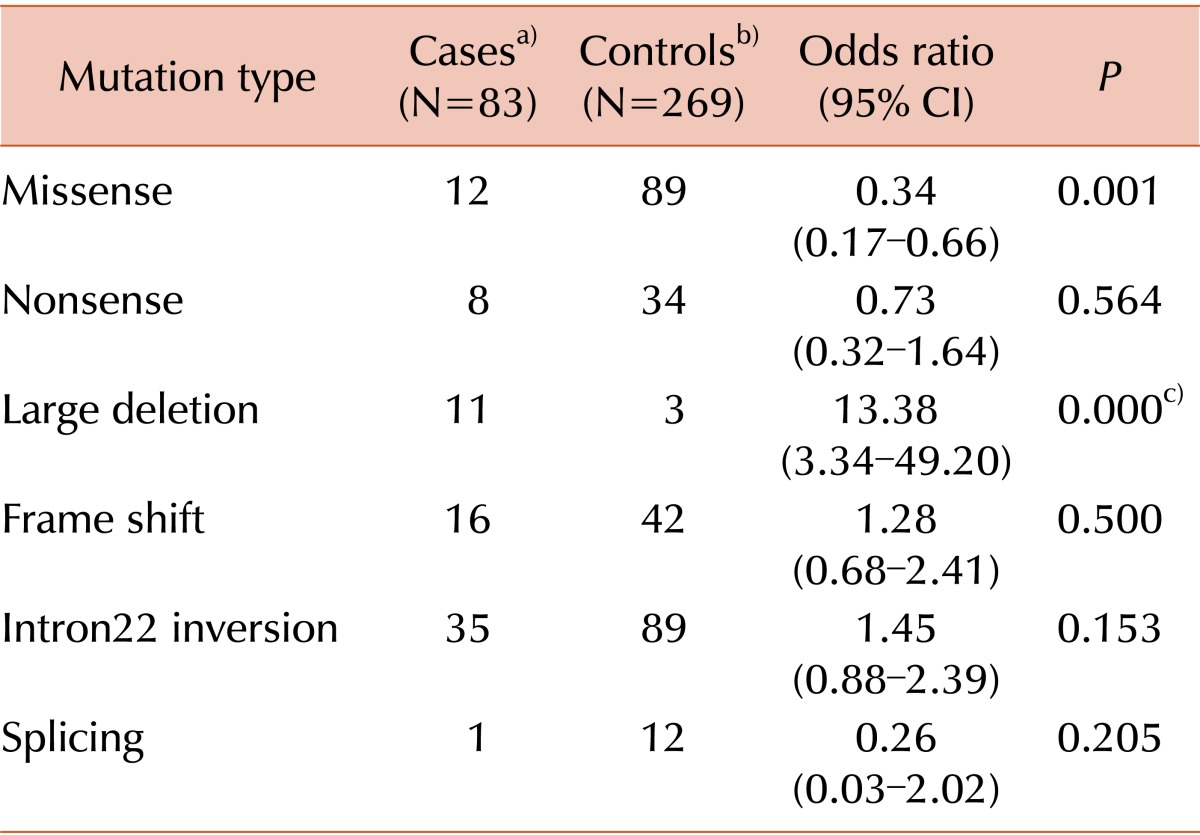
A total of 352 hemophilia A patients, regardless of inhibitor status, underwent direct mutation analysis (Table 4). For the cases, we collected mutation data of patients with transient and persistent inhibitors. For controls, we collected the mutation data of patients who never developed inhibitors. Intron 22 inversion was the most common mutation in both cases and controls. Missense mutation significantly reduced the risk of inhibitor development, whereas large deletion was a high-risk mutation.
Go to : 
DISCUSSION
Inhibitor titers usually begin to increase 2–3 days after exposure to factor VIII, reach a maximum within 7–21 days, and then decrease slowly [17]. The treatment modality depends on the inhibitor titer level. Generally, in low-titer cases, a high-dose infusion of factor concentrate can achieve hemostasis. Patients with high titers must be treated with BPAs. However, ITI can reduce inhibitors permanently by 80–85% and may therefore be preferable. However, several hurdles may hinder the use of ITI. The large injection volume, poor venous access, long duration, and patient compliance may deteriorate ITI performance. The main issue, however, is the high cost of the factor concentrates required for ITI. Even low-dose ITI consumes an amount of factor concentrates equivalent to 5,000 USD per kg of body weight during a single year [16]. Obviously, the overall treatment cost throughout the lifespan of a patient with inhibitors is much more expensive than ITI [3]. However, the extreme costs currently allow the performance of ITI only in well-funded countries.
In this context, the consensus recommendation for ITI, which addresses a waiting period of 1–2 years during which the inhibitor titer may fall below 10 BU/mL, is more understandable [18]. Inhibitor levels of <10 BU/mL can be more easily cured with ITI, and transient inhibitors may largely disappear during that time period [14]. Hence, the consensus recommendation states that unless life-threatening bleedings occur, ITI can be postponed for at least 1–2 years after the development of inhibitors [18]. However, most of the reports regarding long-term courses of inhibitors have included very limited numbers of cases. To our knowledge, this study includes the largest number of subjects in an investigation of the long-term courses of inhibitors.
It is very interesting to determine how long ITI can be postponed and the proportion of patients with transient inhibitors according to the peak inhibitor titer. Once formed, high-titer inhibitors can be present at variable levels even 1–2 years later, without re-exposure to factor VIII. Low-titer inhibitors in cases of mild hemophilia A usually disappear at 4–12 weeks after the discontinuation of factor VIII and do not necessarily reappear upon re-exposure [19]. In the Netherlands, 12 patients with newly developed inhibitors after receiving pasteurized factor VIII achieved inhibitor levels of <2 BU/mL within 8 months [20]. Ören found that 10 (62%) of 16 patients experienced inhibitor disappearance within 24 months, and had titers <2 BU/mL [14]. In this study, 71.4% of all affected patients had transient inhibitors, and 95.8% of patients with inhibitor titers <2 BU/mL became inhibitor-negative within a mean interval of 10.0 months. Sharifian et al. reported a transient inhibitor ratio of only 2.5% among 724 patients with hemophilia in Iran [21], whereas 15.4% of 1,602 hemophilia A patients in Korea had developed transient inhibitors.
The percentage of transient inhibitors is known to be inversely proportionate to the level of inhibitors. Caram et al. analyzed data of 46 hemophilia A patients with inhibitors over a 13-year period [11]. Only 3.4% of patients with inhibitor titers >10 BU/mL became inhibitor negative for 2 years, whereas 55.6% of patients with inhibitor titers <5 BU/mL became inhibitor negative. In this study, 96.5% of patients with inhibitor titers <5 BU/mL eventually became inhibitor negative. However, for patients with inhibitor titers >10 BU/mL, only 29.9% (17/68) became inhibitor negative. Patients with transient inhibitor titers >10 BU/mL remained inhibitor negative for 164 months (median) since the resolution of the most recent inhibitor episode.
Meanwhile, inhibitor recurrence developed in 26.6% of patients with transient inhibitors. Taking into account the time lapse of each episode, close observation for inhibitor recurrence is necessary for at least 3 years, despite the resolution of the inhibitor episode.
Almost all subjects in this study (except for 12 patients) were treated with routine hemostatic dosages, even though they developed high levels of inhibitors and bled frequently. This can be explained by the fact that 60% of the transient inhibitor patients were detected in the early 1990s, when strong constraints were placed on the utilization of medical resources in Korea. Regarding inhibitor titers <2 BU/mL, almost all transient inhibitor patients continued to receive the same dosages of the same clotting factor concentrates that had been used before inhibitor development. Only 1 patient switched to another drug. Further study is required to compare the efficacy of routine hemostatic dosages and large-dose factor replacement.
Also in this study, low-dose ITI was able to eliminate inhibitors in 8 of 77 patients with peak inhibitor titers >10 BU/mL. This is very impressive when taking into account that to date, intermediate-dose ITI (100–200 U/kg) is the recommended protocol for patients with peak inhibitor titers >10 BU/mL in Korea.
Regarding F8 gene mutation, the present study suggests that missense and large deletion mutations might have protective and provocative effects on inhibitor development. Compared to a study by Rosetti et al., the influences of those 2 mutations were identical in present study. Notably, intron 22 inversion, which was significantly risky in their study, was not significantly risky in our study [22]. Although genotype is considered the only proven influence on inhibitor development [23], the relationship between inhibitor development risk and mutation type must be interpreted very cautiously. Specifically, biases related to sample size, inhibitor testing, and ethnic differences may exist. Further study on this subject is needed.
Based on this study, we found that the majority of inhibitors (71.4%) disappeared without ITI within 11.0 months. ITI consumes huge amounts of factor concentrates. Accordingly, if the recorded peak inhibitor titer is <10 BU/mL and no life-threatening bleeding is present, it is worthwhile to wait and observe the clinical manifestations and inhibitor course. However, the inhibitor disappearance rate at peak titers ≥10 BU/mL was only 21.7%. Low-dose ITI can achieve immune tolerance even in patients with very high titers. Moreover, a recent report stated that prompt ITI initiation (≤1 mo after inhibitor detection) for these high titer patients was more successful than later ITI (>1 mo) [24]. It is more reasonable to initiate ITI as soon as possible for patients with inhibitor titers >10 BU/mL.
In conclusion, it is worthwhile to postpone ITI for 11 months unless a patient's peak inhibitor titer is >10 BU/mL. For patients with very high peak inhibitor titers, even low-dose prompt ITI may be preferred for a better outcome.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download